A new study published in Advanced Materials has shown that scientists have taken the next step to develop powerful, rechargeable lithium metal batteries.
The new metal batteries could be safer and simpler to manufacture.
The research was done by a team from the lab of chemist James Tour at Rice University.
Previously, scientists have found that lithium metal anodes charge much faster and hold about 10 times more energy than common lithium-ion anodes used in every electronic device like cellphones and electric cars.
However, the use of high‐energy‐density Li metal anodes in rechargeable batteries is not possible because of dendrite formation that can potentially result in a battery fire.
Last year, the lab has introduced carbon nanotube films that appear to completely halt dendrite growth from lithium metal anodes.
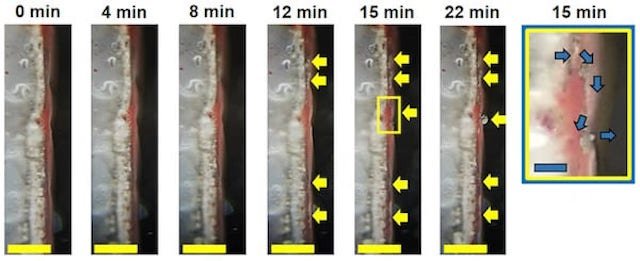
In this study, the researchers made test cells with a coat of red phosphorus on the separator that keeps the anode and cathode electrodes apart.
The phosphorus could act as a spy for management systems used to charge and monitor batteries by detecting the formation of dendrites, protrusions of lithium that can cause them to fail.
With the red phosphorus layer, they observed a sharp drop in voltage when the dendrites contacted the separator.
As soon as a growing dendrite touches the red phosphorus, it gives a signal in the charging voltage. This could warn the battery management system.
The researchers suggest their work provides a further guarantee for battery safety. They offer another layer of protection that should be simple to implement.
The study lead author is Rice graduate student Tuo Wang. Postdoctoral researcher Rodrigo Salvatierra is a co-author.
The study was supported by The Air Force Office of Scientific Research.
Copyright © 2019 Knowridge Science Report. All rights reserved.
Further reading: Advanced Materials.



