Physicists have made a groundbreaking discovery by identifying a brand-new, highly unstable form of aluminum—called aluminum-20—that decays in an extremely rare and dramatic way.
The new isotope sheds three protons as it breaks apart, making it the lightest aluminum isotope ever found and a scientific first in how it decays.
This discovery was published on July 10 in Physical Review Letters by a team from the Institute of Modern Physics (IMP) of the Chinese Academy of Sciences, along with international collaborators.
The researchers used a powerful experimental setup in Germany, at the GSI Helmholtz Center for Heavy Ion Research in Darmstadt, to produce and analyze this exotic nucleus.
Aluminum-20 is far beyond what scientists call the “proton drip line,” a boundary on the nuclear chart where atoms have too few neutrons to stay together.
It has seven fewer neutrons than normal aluminum, making it extremely unstable. Because of this, it doesn’t last long—it decays almost immediately after being created.
The research team used a technique called in-flight decay, where aluminum-20 was created in a high-energy collision and decayed almost instantly as it traveled through a detector.
By closely studying the particles it emitted, the scientists were able to understand how it falls apart.
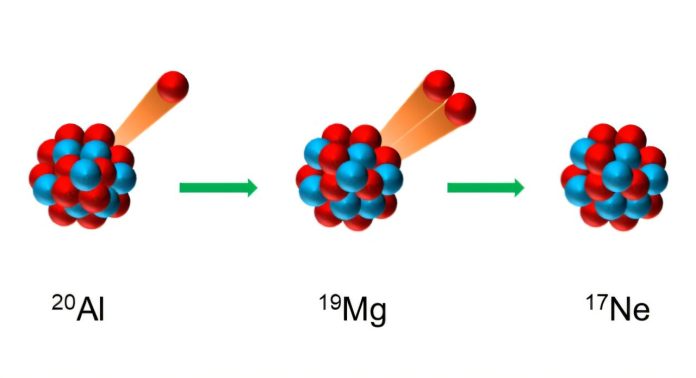
Here’s what they found: aluminum-20 first ejects a single proton, turning into another unstable nucleus called magnesium-19.
But magnesium-19 doesn’t last long either—it emits two protons at the same time. This step-by-step breakdown of one proton followed by two is the first time scientists have seen this kind of three-proton emission from a nucleus.
It’s a big deal because it gives a rare window into the behavior of elements that exist only for tiny fractions of a second.
Even more interesting, the team found that the energy released during this decay was lower than expected. This hints that aluminum-20 might not follow the usual rules of nuclear symmetry, suggesting a break in something called “isospin symmetry.”
Essentially, it behaves differently from its mirror partner neon-20, which should normally be similar. Advanced theoretical models backed this idea, predicting that aluminum-20’s internal structure is likely different from what was expected.
This discovery adds another piece to the puzzle of how atoms work, especially those on the far edge of the nuclear chart where stability breaks down.
So far, scientists have discovered over 3,300 types of atomic nuclei, but fewer than 300 are stable. The rest, like aluminum-20, exist only briefly and help researchers understand the fundamental forces that hold matter together.
This international collaboration included scientists from IMP, GSI, Fudan University, and more than a dozen institutions worldwide.



