A team of chemists at the Hong Kong University of Science and Technology (HKUST) has made a major breakthrough in the field of green chemistry.
They’ve developed a new type of photocatalyst—using tiny particles called quantum dots—that can drive complex chemical reactions using just 1% of the light energy needed by current methods.
This achievement could change how many chemical products are made, from medicines to materials, by making the process cleaner, faster, and much more energy-efficient.
Photocatalysis is a process where light is used to trigger chemical reactions. Scientists have long been interested in using light—especially visible light—as an environmentally friendly energy source for organic chemistry.
Quantum dots, which are nanoscale semiconductor particles, have shown great potential for this kind of work. But so far, they’ve lagged behind traditional small-molecule photocatalysts because scientists didn’t fully understand how to control their behavior.
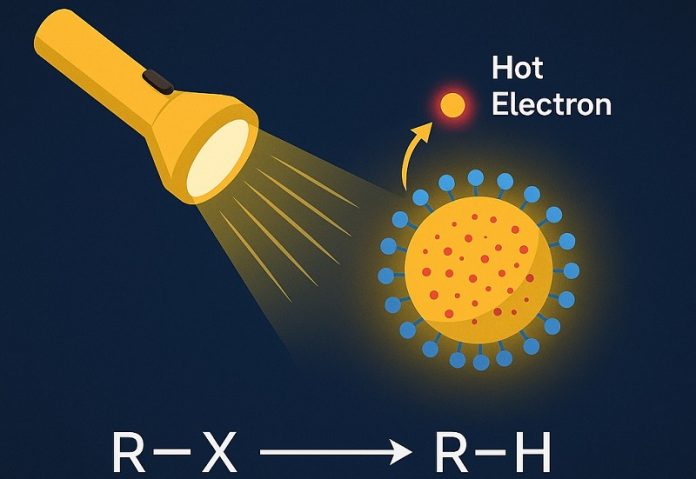
The HKUST team, led by Professor Lu Haipeng from the Department of Chemistry, solved this problem by using specially designed quantum dots made of cadmium sulfide and zinc sulfide (CdS/ZnS) that are doped with manganese ions (Mn²⁺).
These quantum dots absorb visible light and generate what are known as “hot electrons”—high-energy particles that help drive powerful chemical reactions.
What makes this new system so special is how it creates these hot electrons. The researchers used a process called the two-photon spin-exchange Auger mechanism, which allows the quantum dots to produce hot electrons very efficiently under mild conditions.
This is important because it avoids the need for high-powered lasers or extreme environments that are typically required for such reactions.
Thanks to this process, the new catalyst can perform challenging chemical reactions, such as breaking strong carbon-chlorine, carbon-bromine, carbon-iodine, and even carbon-carbon and nitrogen-sulfur bonds.
It can even carry out the Birch reduction, a complex reaction normally requiring harsh conditions. The system works with very low reduction potentials, down to −3.4 volts, showing how powerful the catalyst really is.
Even more impressive, all of this can be done using just a fraction of the light energy used in conventional systems—just 1%.
The researchers can even turn the electron activity on or off by changing the light intensity, allowing for precise control and even programmable reaction sequences.
Professor Lu says this discovery shows the incredible potential of quantum dots to perform complex chemical transformations that traditional photocatalysts cannot achieve.
This innovation opens the door to more sustainable and efficient ways to make useful chemicals, with wide-reaching benefits for industry and the environment.
Source: KSR.



