Scientists have been puzzled by the discovery of collagen, a protein found in bones and connective tissue, in dinosaur fossils that are up to 195 million years old.
Normally, proteins break down much faster—collagen should have a half-life of only about 500 years.
However, a new study from researchers at MIT may explain how this protein could survive for so long.
The research team, led by Ron Raines, a chemistry professor at MIT, found that collagen has a special defense mechanism that protects it from being broken down by water.
Water usually breaks peptide bonds, the links that hold proteins together, through a process called hydrolysis.
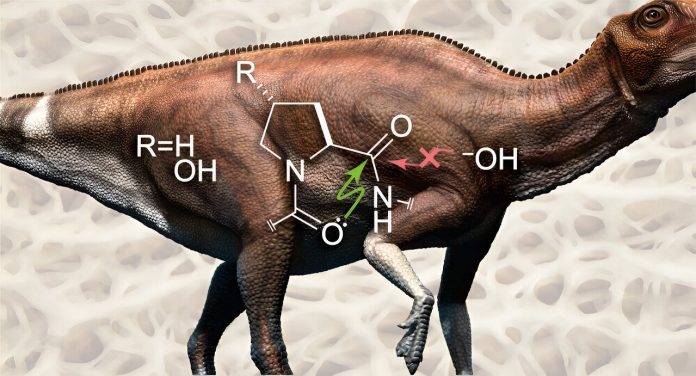
But in collagen, an atomic-level interaction blocks water from doing this, which helps preserve the protein over millions of years.
“We’ve shown that this interaction stops water from attacking the peptide bonds, which is unusual because normally, these bonds have a much shorter lifespan,” says Raines.
He is the senior author of the study, which was published in ACS Central Science.
Collagen is the most common protein in animals, found in bones, skin, muscles, and ligaments. It forms a tough triple helix, where three strands of protein twist together.
This structure makes collagen fibrous and very stable, which is why it’s so important for holding the body together.
Over the last decade, paleontologists have found evidence of collagen in dinosaur fossils, such as an 80-million-year-old Tyrannosaurus rex fossil and another nearly 200-million-year-old fossil from a dinosaur called a sauropodomorph.
Raines’ lab has been studying collagen for many years to understand how its structure helps it function so well. In this new study, the team focused on how the bonds in collagen resist being broken down by water, even after millions of years.
Peptide bonds, which link amino acids together, are key to keeping proteins like collagen intact. These bonds are formed between a carbon atom from one amino acid and a nitrogen atom from the next.
The carbon atom also forms a bond with an oxygen atom, creating what’s called a carbonyl group. This oxygen atom has electrons that don’t form bonds with any other atoms.
Raines and his team found that these free electrons can be shared with the carbonyl group of a nearby peptide bond, creating a sort of molecular shield. This sharing of electrons blocks water from getting in and breaking the bond.
To test this, the researchers created two versions of collagen. One was the normal form, called “trans,” where the bonds are stable. The other version, called “cis,” had bonds that were more open to water. They found that the trans form resisted water and stayed intact, while the cis form allowed water in, and the bonds quickly broke.
Collagen’s triple-helix structure, where the protein is tightly wound from one end to the other, gives it extra protection. “There’s no weak link in collagen, which may explain why it survives for millions of years,” Raines says.
Some scientists have suggested other reasons for collagen’s preservation, such as extreme dehydration of the bones. While this might help, Raines believes the molecular structure of collagen plays a major role in its survival over such long periods.
This discovery provides new insight into why collagen can last for millions of years, helping us better understand the ancient fossils that reveal so much about the distant past.



