Hydrogen is often hailed as the fuel of the future because it produces zero emissions and can store more energy per unit of weight than gasoline.
However, hydrogen’s low density means it takes up a lot of space, making it challenging to store and transport efficiently.
To overcome this, hydrogen must be compressed at extremely high pressures, around 700 bars, which is costly and raises safety concerns.
For hydrogen-powered fuel-cell vehicles (FCVs) to become more common, efficient hydrogen storage is essential.
The U.S. Department of Energy (DOE) has set targets for hydrogen storage systems: they should hold 6.5% hydrogen by weight and 50 grams of hydrogen per liter of storage material.
Meeting these goals would ensure that vehicles can travel reasonable distances without needing frequent refueling.
One promising approach to achieving these targets involves using porous materials like metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and porous organic polymers (POPs).
These materials have tiny pores that can trap and store hydrogen gas.
The goal is to store hydrogen at lower pressures, around 100 bars, to make storage safer and more efficient.
While some materials have met the DOE’s weight-based (gravimetric) targets, they often struggle with space-based (volumetric) capacity.
In practical terms, the volume of the storage system directly impacts how far an FCV can travel, making volumetric capacity even more crucial. Therefore, researchers are working to develop materials that maximize volumetric capacity without sacrificing gravimetric performance.
A new breakthrough comes from a team of researchers led by Professor Fraser Stoddart at The University of Hong Kong and Professor Randall Snurr at Northwestern University.
They focused on organic supramolecular crystals, which are assembled from organic molecules through non-covalent interactions.
These crystals are promising because they are recyclable and could be ideal for hydrogen storage, but designing crystals with the right balance of high gravimetric and volumetric capacities while maintaining stability has been challenging.
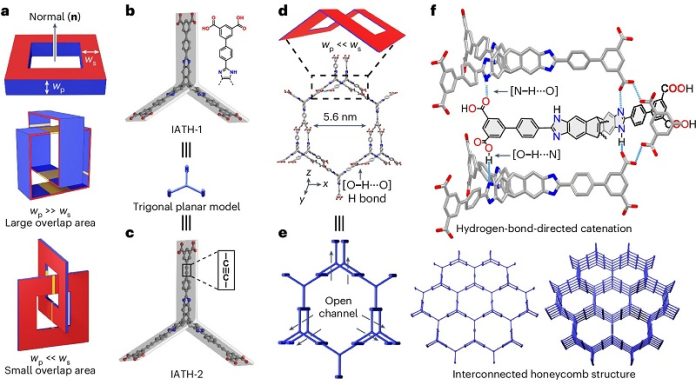
The team introduced an innovative approach called “point-contact catenation.” This method uses hydrogen bonds to guide the assembly of the crystals in a precise way, minimizing surface loss and optimizing pore sizes for hydrogen storage.
Unlike traditional methods that often block pores and reduce surface area, this new strategy preserves the crystal’s porosity and stability.
The result is a supramolecular crystal with record-high gravimetric (3,526 m²/g) and balanced volumetric (1,855 m²/cm³) surface areas.
This crystal also offers excellent material-level volumetric capacity (53.7 g/L) and high gravimetric capacity (9.3% by weight) under practical conditions. Impressively, the crystal surpasses the DOE’s targets for both volumetric and gravimetric storage, although it requires cryogenic temperatures.
This breakthrough highlights the potential of organic supramolecular crystals for hydrogen storage in FCVs. The new point-contact catenation strategy could pave the way for more efficient, stable, and high-capacity hydrogen storage systems, bringing us closer to a future where hydrogen fuel powers our vehicles cleanly and efficiently.
Source: KSR.



