Do you use a smartphone, laptop, or electric car?
Then you probably know the importance of batteries.
But did you know that not all batteries are created equal?
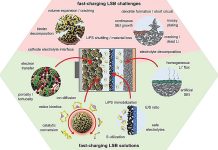
Lithium metal batteries, or LMBs for short, are really promising because they could last longer and store more power.
The problem is, they also have some issues like growing weird spikes called dendrites that can make them less safe and efficient.
Here comes the good news!
A group of scientists from the Shenzhen Institute of Advanced Technology in China, led by Prof. Xue Dongfeng and Dr. Peng Chao, have come up with a really smart way to make these batteries better.
They’ve published their discoveries in a science journal called Matter.
So, what did they do? They used a cool new design method that adds a special layer on the battery’s metal part (known as the anode).
This special layer is made up of carefully chosen molecules and helps solve many of the issues that have been bugging lithium metal batteries.
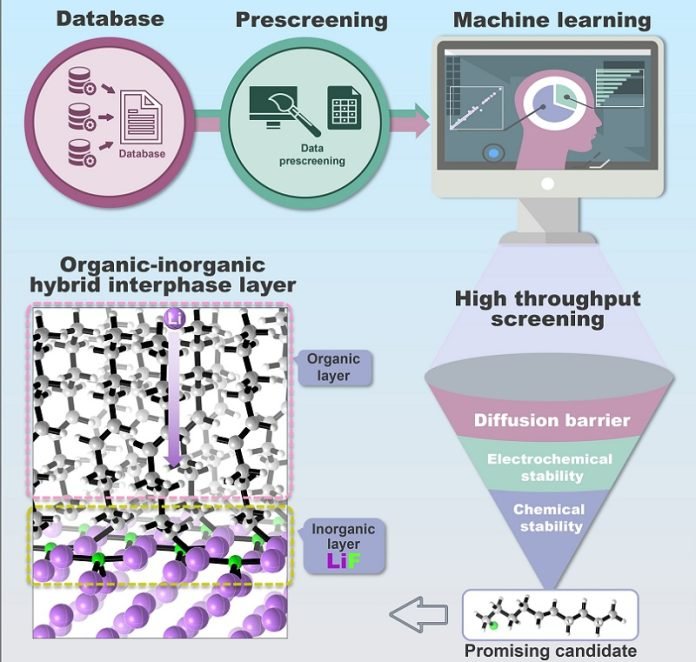
The really cool part is how they chose these molecules. Instead of doing it the slow, traditional way, they used a high-tech method involving machine learning.
This is basically a way to let computers sift through lots of data to pick the best options quickly. They even created a system that automatically picks good molecules from a big database called PubChem.
With this smart design, the special layer provides two main benefits. First, it helps the lithium in the battery spread out evenly, stopping those problematic spikes (dendrites) from growing. Second, it makes sure the lithium interacts well with the stuff in the battery called electrolyte, which is key to making the battery work well.
The scientists didn’t stop there. They dug deep to understand how different parts of the molecule affect the battery’s performance.
They found that the electric charge in the molecules could be used to predict how well the protective layer will work. The team ended up with a list of eight top-performing molecules that could be used to make this special layer.
Why should we care? Well, with this new layer, lithium metal batteries could become a lot safer and more efficient.
That means your gadgets could last longer, and electric cars could drive further on a single charge. Plus, this is a huge step towards making these advanced batteries a real option for everyday use.
Dr. Peng, who is one of the main authors of the study, said, “Our study opens up new possibilities for the development of more efficient and safer lithium metal batteries.”
So, the next time you charge your phone or drive an electric car, remember that scientists are working hard to make those batteries even better!
Follow us on Twitter for more articles about this topic.
Source: Chinese Academy of Sciences.