Imagine if your phone or laptop battery could last longer and recharge quicker.
That’s what a team of scientists, led by Prof. Hu Linhua at the Hefei Institutes of Physical Science, Chinese Academy of Sciences, is working on.
According to their study published in Renewables, they’ve made some big leaps forward with a material known as anatase titanium dioxide (or TiO2 for short) which is used in batteries.
What’s so special about this material?
Well, it’s stable, cheap, environmentally friendly, and safe. That’s why it’s being looked at as a good choice for the part of a lithium-ion battery known as an anode.
Anodes are one of the key parts of a battery, where electricity comes from.
But there’s a problem with TiO2.
It’s a type of material called a semiconductor, which means it’s not very good at conducting electricity or allowing ions (which are tiny charged particles) to move around in it.
This limitation leads to batteries that can’t store much power and can’t recharge quickly or effectively. That’s a big roadblock to using it in practical applications.
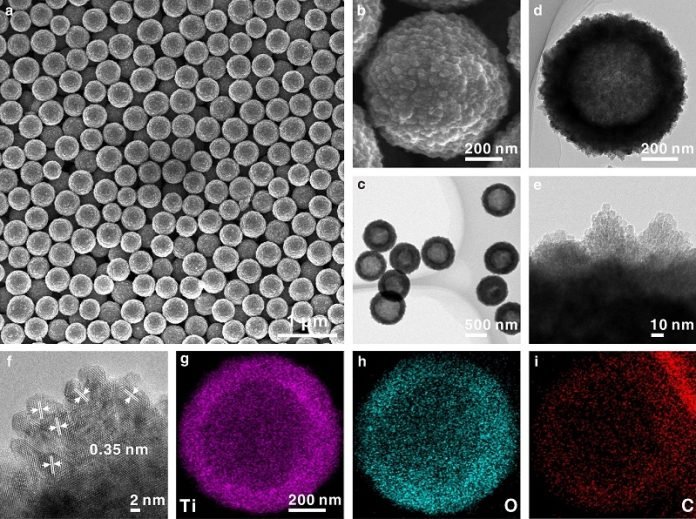
The scientists, however, found a way to improve the performance of TiO2. They added a substance called carbon and made small defects (oxygen vacancies) in it.
Then they formed the TiO2 into hollow spheres, which they called HS-TiO2. They compared these with white TiO2 hollow spheres (W-TiO2) that were fully oxidized, meaning they didn’t have the added carbon or oxygen vacancies.
They found that the HS-TiO2 had a smaller bandgap. Without getting too technical, this means it’s easier for electricity to flow in it. Also, the changes they made resulted in the presence of localized electrons. These electrons made it easier for lithium ions to move around, which sped up the rate of ion movement.
So, what does all this mean? Basically, when lithium ions were added and taken away (which happens when a battery is charged and then used), the HS-TiO2 worked better than the W-TiO2.
This implies that batteries using HS-TiO2 could recharge quicker and hold more power.
The researchers could also control the internal structure of these hollow spheres by changing the duration of a certain type of reaction called a solvothermal reaction.
They could make the spheres solid, porous, or keep them hollow, all while keeping their outer shape the same. This level of control over the structure can help them understand how different structures might affect battery performance.
The findings from this study give us a big step forward in understanding how we can use carbon and oxygen vacancies in TiO2 to make better batteries.
It opens up new possibilities for exploring and optimizing the use of these hollow spheres and similar materials.
It’s pretty exciting stuff – who knows, it might just lead to the next big breakthrough in battery technology!
The study was published in Renewables.
Follow us on Twitter for more articles about this topic.
Source: Chinese Academy of Sciences.