For high-voltage lithium batteries using layered transition metal oxide cathodes, the chemical degradation of the battery’s electrolytes results in rapid decay of its capacity.
This poses substantial challenges to practical applications of this potential next generation of lithium batteries.
Researchers from the Qingdao Institute of bioenergy and bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) have been inspired by the way that organisms tackle the aging effects of highly reactive oxygen and free radicals to solve this problem.
The study was published in the Journal of the American Chemical Society.
In a classical battery’s electric circuit, the electrodes—one negative (the cathode) and one positive (the anode)—are the conductors that make contact with the non-metallic part of a circuit (the electrolytes).
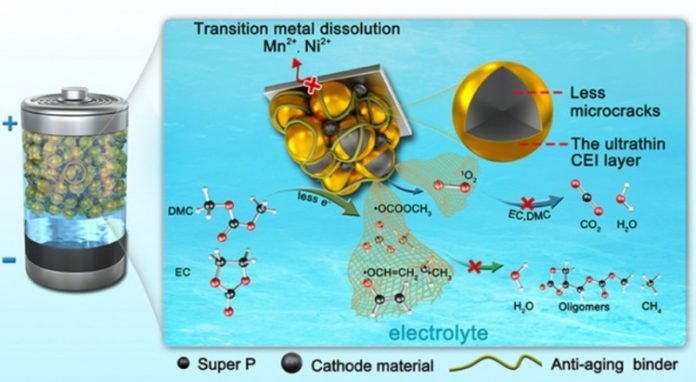
The chemical degradation of the electrolytes in lithium batteries with transition-metal oxides is caused during the cycling of the circuit by oxidation of highly reactive oxygen and by an attack of free radicals—any atom, molecule, or ion with an unpaired valence (outer layer) electron.
Some strategies aimed at tackling the problem mostly involve clever engineering tricks at the surface atomic layer of the materials, or of using solid rather than liquid electrolytes.
The aim here is building up a physical barrier to slow down the rate of electrolyte decomposition. But even a slowing down does not substantially suppress their chemical degradation over time.
In nature, such degradation occurs pretty much everywhere, since oxygen is one of the elements most capable of attracting electrons from other atoms and molecules. An apple browning, a lump of iron rusting and your skin aging are all in part a product of oxidation ‘damage’.
And nature has come up with all sorts of solutions to counter this problem. Organisms often produce different types of enzymes that work to scavenge active oxygen and free radicals to alleviate the issue.
“So, we thought, why not just try to replicate what nature already does, and put it inside a battery instead?” said CUI Guanglei from QIBEBT, a lead researcher on the study.
Inspired by these organismal anti-oxygen coping mechanisms, the researchers developed a photostabilizer—a fairly simple, anti-aging binder additive to the electrolyte that can scavenge the singlet oxygen atoms and free radicals as occurs.
Through experimental investigation and theoretical calculation, the researchers found that this bio-inspired oxygen scavenging mechanism in layered transition metal oxides-based lithium batteries delivered superior electrochemical performance, even under elevated temperatures.
“This heralds a new paradigm for manipulating the cathode and electrolyte chemistry of all sorts of rechargeable batteries involving chemical degradation of electrolyte,” Prof. CUI added.
Following on from the photostabilizer success, the researchers aim to commercialize high-voltage layered oxide cathode-based lithium batteries with their bio-inspired anti-aging binder as the next generation of energy storage devices beyond traditional lithium-ion technology.



