Physicists at the Massachusetts Institute of Technology (MIT) have developed a revolutionary way to look inside an atom’s nucleus — without needing a massive particle accelerator.
Instead, they used the atom’s own electrons, trapped inside a molecule, as tiny messengers to probe its core.
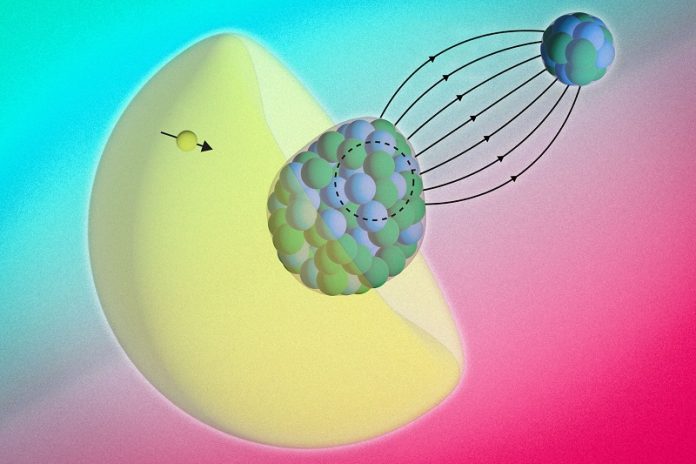
In a study published in Science, the researchers measured the energy of electrons orbiting a radioactive radium atom that was chemically paired with a fluoride atom to form a molecule called radium monofluoride.
This molecule created a powerful microscopic environment, where the radium’s electrons were squeezed so tightly that some briefly penetrated the nucleus itself.
When the electrons emerged, they carried subtle changes in their energy — “messages” revealing information about the nucleus’s inner structure.
This discovery provides scientists with a new, tabletop method to explore one of the most mysterious regions in physics: the atomic nucleus.
Traditionally, experiments that study atomic nuclei require enormous facilities that accelerate particles over kilometers to smash into atoms. The MIT team’s molecular approach achieves similar insights using equipment that fits on a laboratory bench.
By precisely measuring the electrons’ energies inside radium monofluoride, the team found a small but unmistakable energy shift — evidence that the electrons had briefly interacted with the protons and neutrons inside the nucleus.
“We now have proof that we can sample inside the nucleus,” said Professor Ronald Fernando Garcia Ruiz, who led the study. “It’s like being able to measure a battery’s electric field not just outside, but inside the battery itself.”
The experiment opens a new way to map the magnetic distribution inside the nucleus — how protons and neutrons, each with their own tiny magnetic fields, align and move.
Understanding this could help answer one of the biggest puzzles in modern physics: why our universe contains so much more matter than antimatter.
According to the leading theory of physics, the Standard Model, the Big Bang should have produced equal amounts of matter and antimatter.
But everything we see — from stars to galaxies to ourselves — is made of matter.
Scientists believe this imbalance may be due to subtle violations of “fundamental symmetries” inside atomic nuclei, and radium’s unusual structure makes it a prime candidate to test that theory.
Unlike most atomic nuclei, which are spherical, the radium nucleus has an asymmetric “pear shape.”
This shape amplifies any tiny symmetry-breaking effects, making them easier to detect. “Radium is predicted to be an amplifier of this symmetry breaking,” said Garcia Ruiz. “That makes it an exceptional system to study.”
Because radium is radioactive and short-lived, the researchers could only create the molecules in tiny quantities, requiring highly sensitive detection techniques.
They trapped and cooled the molecules in vacuum chambers and used laser light to measure the electrons’ energy with extreme precision. The detected energy shift — only about one millionth of the laser’s photon energy — confirmed that electrons had indeed entered the nucleus.
“Normally, we study interactions between electrons and the nucleus from the outside,” said lead author Shane Wilkins, a former MIT postdoctoral researcher. “But here, the measurements didn’t add up unless we assumed the electrons had gone inside. That was the key clue.”
The team now plans to cool the molecules even further and control their orientation to create detailed maps of the forces and symmetry properties inside the radium nucleus.
“Radium-containing molecules are predicted to be exceptionally sensitive tools for studying the fundamental symmetries of nature,” Garcia Ruiz said. “And now, we finally have a way to look inside.”



