Lithium powers many parts of our modern world—from smartphones and laptops to electric vehicles and military gear.
As demand for lithium continues to soar, countries are becoming increasingly concerned about having reliable and affordable sources.
Right now, most lithium is mined from hard rock or pumped from salt lakes, mostly located in just a few countries. This limited supply poses a risk for global supply chains.
But scientists at the U.S. Department of Energy’s Argonne National Laboratory may have found a promising new way to extract lithium more cheaply and efficiently—from water.
Their research, done in partnership with the University of Chicago, was recently published in the journal Advanced Materials.
Although most of Earth’s lithium is dissolved in seawater and underground saltwater reserves, getting it out has been extremely difficult and expensive. That’s because lithium is mixed in with other minerals like sodium and magnesium, which are often more concentrated and harder to separate.
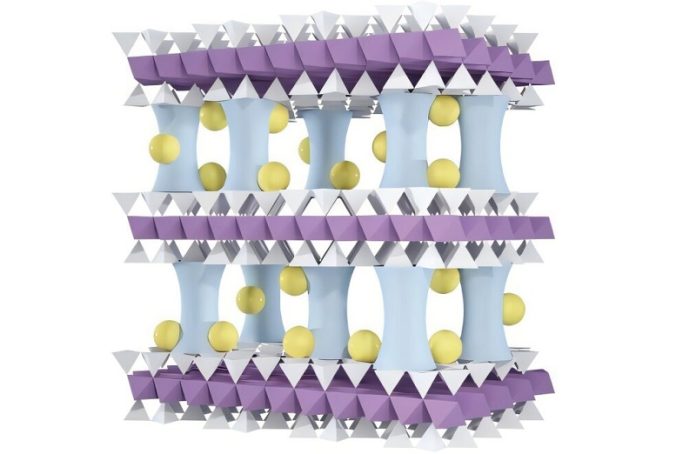
To tackle this challenge, the Argonne team developed a cutting-edge membrane that acts like a smart filter. It’s made from vermiculite, a naturally occurring clay that costs just around $350 per ton. The team found a way to peel the clay into ultra-thin sheets—only a billionth of a meter thick—and stack them into a filter-like structure. These ultrathin sheets are so slim they’re considered two-dimensional.
However, the clay fell apart quickly in water, lasting only about 30 minutes. To fix this, the scientists inserted tiny aluminum oxide pillars between the layers, similar to the support beams in a high-rise building under construction. This structure keeps the layers from collapsing and neutralizes the membrane’s surface, making it easier to fine-tune.
The next step involved adding sodium ions, which carry a positive electric charge. These ions changed the membrane’s surface to positive as well. Since magnesium ions have a higher charge than lithium, the membrane now repels magnesium more strongly, allowing lithium ions to pass through more easily.
By adding even more sodium ions, the team shrank the size of the membrane’s pores. This makes it possible to filter ions not just by their electric charge, but also by size—letting smaller ones through and capturing the lithium.
This dual-filtering ability could open the door to extracting lithium from seawater or saltwater lakes in a way that’s much cheaper and less harmful to the environment. It might also help recover other important materials like cobalt and rare earth elements or even clean up contaminated water.
As demand for lithium grows, this low-cost clay membrane could become an important tool in securing the materials needed for a cleaner, tech-driven future.



