Scientists at the University of Sydney have developed a groundbreaking new way to make ammonia—one of the world’s most essential chemicals—using a method that mimics lightning.
This innovation could lead to a cleaner, greener future for agriculture and energy.
Ammonia is a key ingredient in fertilizer, which helps grow almost half the world’s food. It’s also being explored as a clean energy source and hydrogen carrier.
But the current method of producing it, known as the Haber-Bosch process, is energy-intensive and relies heavily on fossil fuels.
It creates a massive carbon footprint and must be done on a large scale, near cheap natural gas sources, to be cost-effective.
The Sydney team, led by Professor PJ Cullen from the School of Chemical and Biomolecular Engineering, has been working for six years on a more sustainable alternative.
Their new approach uses electricity to excite molecules in air, creating plasma—like artificial lightning—which then helps turn nitrogen from the air into ammonia gas.
This method skips the need for high temperatures, fossil fuels, and large-scale infrastructure.
What makes this discovery stand out is that previous green ammonia efforts by other labs produced ammonia in liquid form (called ammonium), which still required further processing.
The Sydney team, however, directly created ammonia gas, which is ready for immediate use. This makes the process simpler and potentially much more efficient.
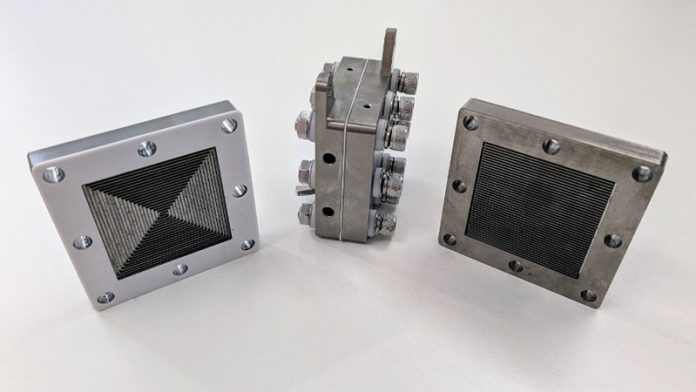
At the heart of their system is a membrane-based electrolyzer, a compact silver device where the conversion to gaseous ammonia takes place.
The process involves two main steps: first, plasma created from excited air molecules; second, passing these molecules through the electrolyzer to form ammonia.
Ammonia, made up of three hydrogen atoms and one nitrogen atom, has other promising uses beyond fertilizers. It can act as a clean fuel because it doesn’t release carbon dioxide when used. It’s also an effective way to store and transport hydrogen, which could help power industries like shipping—one of the world’s largest polluters.
Professor Cullen says this research could help make “green ammonia” a real possibility. It would allow smaller-scale, decentralized ammonia production—cutting the need for massive industrial plants and long-distance transport.
The team has already proven that the plasma part of the process is energy efficient and scalable. Their next challenge is to make the electrolyzer equally efficient so the entire method can compete with the traditional Haber-Bosch process.
With this breakthrough, the future of ammonia production might shift from smoke-spewing factories to clean, lightning-powered labs—and that’s a spark of hope for the planet.



