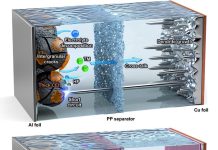
Scientists have developed a new way to create battery electrolytes—the part of a battery that moves charged particles between its terminals—using a single-step process.
This breakthrough could make batteries more efficient, longer-lasting, and cheaper to produce.
The research, led by scientists at the University of Chicago’s Pritzker School of Molecular Engineering, was published in Chemistry of Materials.
Battery electrolytes come in two main types:
- Solid inorganic electrolytes – These allow electricity to flow quickly, but they are brittle and hard to work with.
- Polymer electrolytes – These are flexible and easy to shape, but they don’t conduct electricity as well.
Scientists have tried mixing the two to get the best of both worlds, but the results have been inconsistent.
The biggest challenge is getting them to blend properly, as traditional methods often lead to lumpy, uneven mixtures that reduce battery efficiency.
The ‘one-pot’ solution
Instead of making the materials separately and mixing them later, the research team developed a one-pot technique—meaning both materials are made together in the same vessel.
This ensures a perfect, even mix while also saving time and money in production.
“When you use the one-pot method to make lithium metal batteries, the results are much better than traditional mixing methods,” said Assistant Professor Chibueze Amanchukwu, who led the study.
The researchers focused on lithium batteries, which power electric vehicles (EVs), smartphones, and energy storage systems.
However, the method could also be applied to sodium batteries, which are a promising alternative to lithium because sodium is cheaper and more abundant.
The technique also has potential uses in electronics, industrial coatings, sealants, and wearable technology.
“If you need a material that is flexible and can twist and turn, like in wearable electronics, this method lets us engineer the polymer to give it that ability,” said first author Priyadarshini Mirmira.
While the process works well in the lab, scaling it up for mass production will require solving a few challenges:
- Temperature control – The mixture gets hot as it reacts. Factories will need precise control to keep the temperature just right.
- Air-free conditions – The reaction needs to take place in argon or another inert gas to prevent contamination, which is easier in a lab than on a factory floor.
Once these challenges are solved, the one-pot method could lead to cheaper, more efficient hybrid battery materials that are easier to produce on a large scale.
“We wanted to create a fully integrated inorganic-polymer material, and we succeeded,” said Mirmira. “This discovery opens the door to better batteries and smarter materials for the future.”