Microplastics, tiny plastic particles, are found almost everywhere—in the air, soil, oceans, and even inside our bodies.
These fragments, created by the breakdown of larger plastic items or from synthetic fibers in clothing, are a growing environmental problem.
Even smaller nanoplastics, which are invisible to the naked eye, are even more dangerous.
They can pass through biological barriers and harm vital organs. Scientists recently found nanoplastics in the human brain, raising serious concerns.
Now, researchers at the University of São Paulo (USP) in Brazil have created a promising and affordable way to remove micro- and nanoplastics from water using nanotechnology.
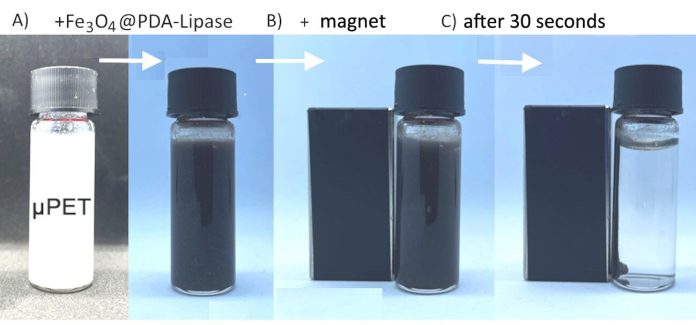
Their study, published in the journal Micron, introduces a technique that uses magnetic nanoparticles coated with a special material to capture and remove plastic particles.
The process starts with magnetic nanoparticles made from black iron oxide (Fe3O4). These particles are coated with polydopamine, a substance inspired by the natural adhesive properties of mussels.
Polydopamine sticks to plastic fragments in the water, allowing the magnetic nanoparticles to attach to the plastic. Then, the combined particles can be easily removed from the water using a magnet.
“Polydopamine works like glue, sticking firmly to plastic particles in the water,” explained Dr. Henrique Eisi Toma, a chemistry professor at USP and one of the study’s authors.
“This method helps remove micro- and nanoplastics effectively, especially in water treatment systems.”
The researchers aren’t stopping at just removing plastics. They are also exploring ways to break down plastics into smaller, reusable molecules.
This involves using enzymes like lipase, which can decompose common plastics such as polyethylene terephthalate (PET). PET, used in items like plastic bottles, is a major pollutant and can release toxic byproducts when it degrades.
Lipase breaks PET into its basic components, such as terephthalic acid and ethylene glycol, which can then be reused to create new plastic materials. This approach not only cleans water but also contributes to recycling plastic in a sustainable way.
“Our goal is to remove plastic from water and help recycle it,” said Dr. Toma. “This method could be expanded to target other types of plastics, like nylon, using specific enzymes.”
Plastics, made from fossil fuels, are widely used because they are cheap, durable, and lightweight. However, their extensive use has created an environmental crisis. Even bioplastics, which are made from renewable materials, can break down into microplastics and interact with living organisms in harmful ways.
Disturbingly, bottled water may be more contaminated with microplastics than tap water. Tap water goes through filtration and other treatments, while bottled water doesn’t. If the source of bottled water is already polluted with microplastics, these particles can end up in the bottle.
The USP team’s innovative solution is an important step forward, but the problem of microplastics remains massive and complex. Dr. Toma hopes their work inspires other researchers to find additional solutions and urges governments to address this issue seriously.
Microplastics and nanoplastics are a threat not only to the environment but also to human health. The new nanotechnology developed at USP offers hope for tackling this challenge, making water safer and contributing to a cleaner, more sustainable future.



