A team of researchers at the University of Twente, led by Dr. Marco Altomare, has discovered a way to make green hydrogen production more efficient by using less precious metal, such as platinum, without losing performance.
Their findings, published in the journal Advanced Functional Materials, showcase a method that could make hydrogen energy more sustainable and affordable.
The world is urgently looking for sustainable energy sources to combat climate change and the ongoing energy crisis.
Green hydrogen is a key player in this transition, but making it on a large scale requires efficient and durable technology.
Currently, polymer electrolyte membrane (PEM) water electrolyzers and fuel cells rely on precious metals like platinum and iridium to produce hydrogen efficiently. However, these metals are expensive and scarce, limiting the growth of hydrogen technologies.
The U.S. Department of Energy (DOE) has set a goal to improve hydrogen production efficiency by five to ten times by 2026, while using less than 20% of the current amount of precious metals.
This means reducing the use of platinum and iridium to less than 0.6 mg/cm², a significant scientific and technological challenge.
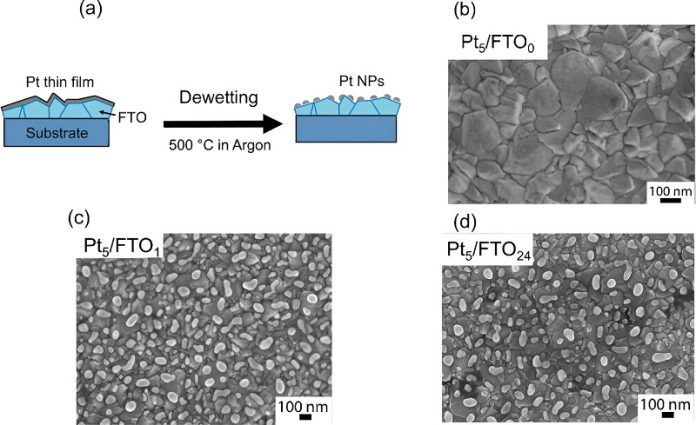
Dr. Altomare’s team tackled this challenge by studying platinum as a model catalyst.
They used a combination of physical vapor deposition (PVD) and controlled thermal treatments, known as solid-state dewetting, to create electrodes that are highly active and durable, yet use much less precious metal.
Their preliminary experiments suggest that they can reduce the amount of platinum needed by five times without any loss in hydrogen production.
Ph.D. researcher Shreyas Harsha, who is leading the project, explained, “With our approach, we can potentially reduce the amount of precious catalyst needed by five times without sacrificing H2 generation performance.”
Dr. Altomare added, “Our method is completely chemical-free, safer, and produces no waste of precious catalyst precursor.
It is also scalable, as similar thin film deposition methods are already used in various industrial applications. Our facilities at the University of Twente can coat catalyst layers on surfaces up to a few hundred square centimeters.”
The team’s next goal is to collaborate with Dutch research centers and companies to test their electrodes under industry-relevant conditions.
They aim to demonstrate and validate efficient and stable water electrolysis with noble metal loadings reduced to less than 0.5 mg/cm².
If successful, this breakthrough could significantly impact the future of green hydrogen production and sustainable energy, making it more accessible and affordable.



