Researchers at The University of Texas at Austin have created a new method to store carbon captured from the atmosphere, which works much faster than current techniques and doesn’t use harmful chemicals.
This groundbreaking research was published in ACS Sustainable Chemistry & Engineering.
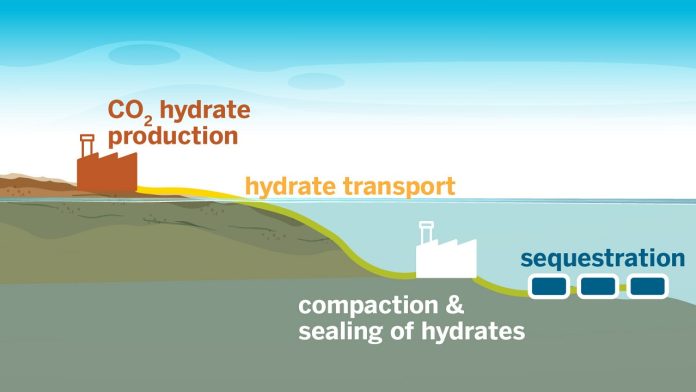
The team developed a way to rapidly form carbon dioxide hydrates, which are unique ice-like materials that can trap carbon dioxide in the ocean, preventing it from being released into the atmosphere.
This technique could play a crucial role in tackling the enormous challenge of safely removing large amounts of carbon from our atmosphere.
Professor Vaibhav Bahadur, who led the research, explained, “Hydrates offer a universal solution for carbon storage.
To make them a major part of our carbon storage efforts, we need the technology to grow them quickly and at scale. We’ve shown that we can rapidly grow hydrates without using any chemicals that would negate the environmental benefits.”
Carbon dioxide is the most common greenhouse gas and a significant driver of climate change. Carbon capture and storage (CCS) involves removing carbon from the atmosphere and storing it permanently.
This process is essential for reducing the carbon footprint and decarbonizing our planet.
Currently, the most common method for storing carbon involves injecting it into underground reservoirs.
While this technique helps trap carbon and can boost oil production, it has significant drawbacks, including potential leakage, groundwater contamination, and seismic risks. Additionally, many regions lack the geological features necessary for this type of storage.
Hydrates could become the preferred method for large-scale carbon storage if some of these issues can be resolved.
Until now, forming these carbon-trapping hydrates was slow and energy-intensive, limiting their large-scale use.
However, the new method developed by the researchers increases the rate of hydrate formation by six times compared to previous methods, and it’s chemical-free, making it more practical for mass-scale carbon storage.
The secret to their success lies in using magnesium as a catalyst, which eliminates the need for chemical promoters.
This is combined with a high flow rate of bubbling CO2 in a specially designed reactor. The technology works well with seawater, simplifying the process since it doesn’t require complex desalination to create fresh water.
“Hydrates are an attractive carbon storage option because the seabed offers stable conditions that protect them from decomposing,” Bahadur said. “This technology makes carbon storage accessible to every country with a coastline, making it more feasible globally and bringing us closer to a sustainable future.”
Beyond carbon storage, this breakthrough has potential applications in desalination, gas separation, and gas storage, offering versatile solutions for various industries.
The researchers and the university have filed for patents related to this technology and are considering starting a company to commercialize it. This new method could be a game-changer in the fight against climate change and help us achieve a more sustainable future.



