Scientists have made a groundbreaking discovery that could revolutionize the battery world, potentially making our gadgets last much longer and reducing environmental harm.
Led by UCLA’s Xiangfeng Duan and Philippe Sautet, a team of chemists has cracked the code on a complex chemical reaction that’s key to advancing lithium-sulfur batteries.
These batteries promise to store up to ten times more energy than the lithium-ion batteries we use today, and they could be much cheaper and less damaging to the planet.
Lithium-ion batteries, which power everything from smartphones to electric cars, rely on cobalt oxide for their cathodes.
Cobalt is not only pricey but also sourced in ways that can hurt people and the environment. Lithium-sulfur batteries could be the solution we’ve been waiting for, as they use sulfur instead of cobalt. Sulfur is not just abundant and environmentally friendly; it’s also incredibly cheap, costing less than 1% of what cobalt does.
However, there’s been a significant obstacle: the battery’s chemistry is complicated. Specifically, the process of reducing sulfur to make the battery work involves numerous steps and can lead to unwanted side reactions that shorten the battery’s life.
The research team from UCLA has now mapped out this complex reaction for the first time. By understanding exactly how the reaction unfolds, they can pinpoint which steps to enhance for better battery performance.
Their work, published in the prestigious journal Nature, lays the groundwork for making lithium-sulfur batteries more efficient, long-lasting, and practical.
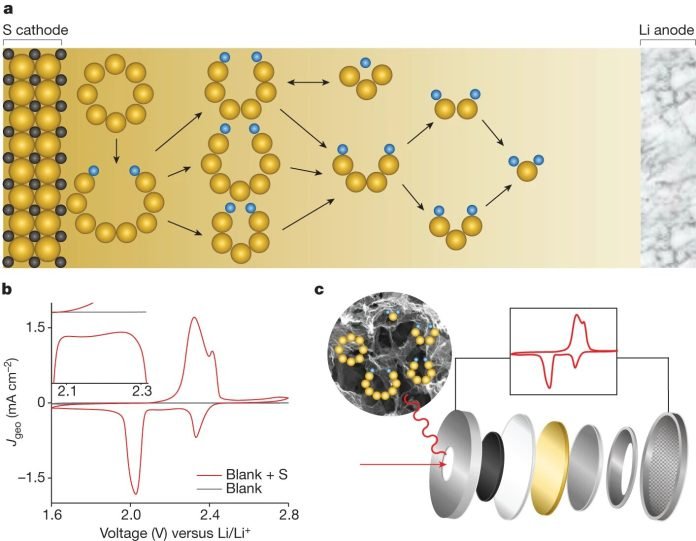
In a lithium-sulfur battery, converting sulfur into lithium sulfide—the action that powers the battery—requires a detailed series of steps involving 16 electrons and creates a variety of intermediate products.
Until now, figuring out how to manage this process for the best battery performance has been a significant challenge because of the reaction’s complexity.
One of the biggest problems with current lithium-sulfur batteries is that some of the intermediate sulfur compounds tend to move from one part of the battery to another, wasting sulfur and lithium and leading to a loss of energy and storage capacity. The UCLA team’s findings offer a roadmap to prevent this from happening by identifying the crucial steps and products in the reaction.
Their research showed that one particular intermediate, Li2S4, plays a central role in the battery’s operation. They also discovered that using carbon-based electrodes, treated with sulfur and nitrogen, can help convert this intermediate into the final product more efficiently, reducing waste and improving the battery’s life.
Furthermore, the team found that another intermediate, Li2S6, doesn’t directly help generate electricity but instead contributes to the unwanted movement of compounds within the battery. This insight is crucial for designing batteries that minimize energy loss.
Duan explained, “Our study not only explains the battery’s chemistry in detail but also shows how tweaking the electrode material can lead to significant improvements.” Sautet added, “This blend of battery technology and catalysis science opens exciting new possibilities for high-capacity, fast-charging energy devices.”
This breakthrough could lead to batteries that make our electronics last longer and charge faster, all while being more affordable and less harmful to the environment. It’s a big step forward in our quest for sustainable energy solutions.



