Everyone loves a gadget.
Be it our trusty smartphone, a laptop, or perhaps an electric vehicle, these devices simplify and energize our daily life.
At the heart of many of these modern marvels lie lithium batteries. However, as we yearn for more advanced, swift, and enduring devices, the question arises: can our current batteries keep up?
A group of sharp minds at Hong Kong Polytechnic University led by Zhijie Wang, a Postdoctoral Research Fellow, and Biao Zhang, an Associate Professor, dove deep into this question, examining the core of lithium batteries – the electrolytes – to find a path forward.
Their insights were recently shared with the world in a paper published in Energy Materials and Devices.
A Peek Inside the Battery
To grasp the researchers’ findings, let’s take a moment to understand a bit about how lithium batteries function. Envision a battery as a bustling city.
It comprises two large terminals (anodes and cathodes, aka the positively and negatively charged ends), and an electrolyte, which acts like a highway for charged atoms (ions) to travel between the two ends.
When we charge and use a battery, these ions are on the move, hopping back and forth between the two ends, and that movement powers our devices.
The Pivotal Role of Electrolytes
The electrolyte is more than a mere pathway. It’s a critical component that determines how well the battery performs, how long it lasts, how safe it is, and how quickly it can charge our devices.
Commonly, the electrolyte involves a lithium salt solution, which assists in creating a flow of ions.
Now, the way these electrolytes deal with lithium ions (the busy travelers on our highway) and other elements can differ.
Traditionally, lithium ions detach themselves from associated particles (salt anions) in what we might imagine as a straightforward route for the ions.
However, in more recent designs, these lithium ions pair up with the salt anions during their journey, which has been shown to enhance battery performance.
A Softer, Weaker Approach to Power
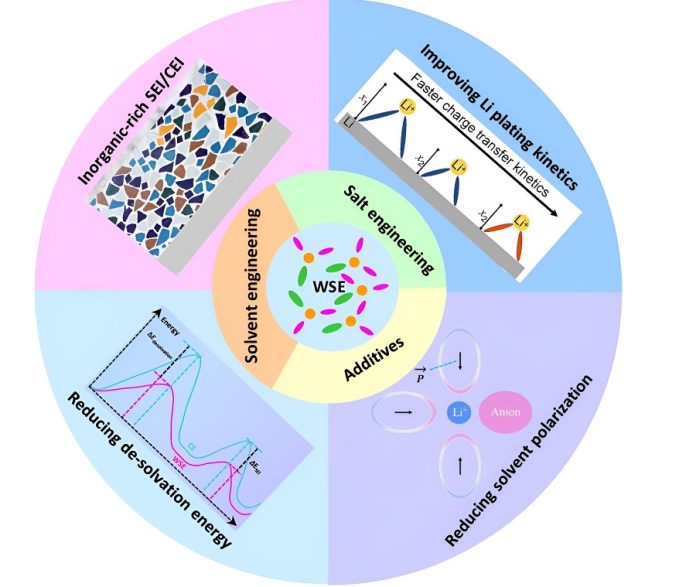
Wang and Zhang shed light on a relatively new player in the game: weakly solvating electrolytes. This kind of electrolyte does something different.
It doesn’t tightly bind the lithium ions; instead, it loosely coordinates them with the solvent molecules and the negatively charged salt anions.
Imagine a bustling highway in a tight, strict city where every car (ion) has to follow a rigid path. That’s a traditional electrolyte.
Now, consider a more flexible, open city, where cars can take slightly varied, flexible routes to their destination.
That’s the scenario in weakly solvating electrolytes. This approach, despite sounding a bit lax, can significantly enhance various properties of lithium batteries, like ensuring better safety, allowing faster charging, and providing reliable performance even in colder temperatures.
Looking Ahead with ‘Weak’ Power
Wang and Zhang dove through piles of research papers on this intriguing kind of electrolyte and realized that while it has garnered much attention, there’s still a lot we need to establish in terms of designing these “weak” electrolytes effectively and understanding them thoroughly.
Creating weakly solvating electrolytes is like devising a city plan where the right balance must be struck between the various routes and paths taken by the ions.
Wang and Zhang believe this “weaker” and more flexible strategy could be applied not only to lithium batteries but also to batteries made with other materials like sodium, potassium, magnesium, or zinc.
The goal? Make batteries that power our future effectively and sustainably. By honing in on the best designs for weakly solvating electrolytes, the researchers aim to simplify the creation process, enhance the results, and make it economically viable.
Through this, they hope to contribute a sturdy foundation upon which the future of powerful, reliable, and safer batteries can be built, lighting up our devices and lives in a more robust and enduring manner.
In a nutshell, while our devices become increasingly sophisticated, researchers like Wang and Zhang are ensuring the heart that powers them—our batteries—are not just keeping pace but are ahead of the game, paving the way for a brighter, energized future.
Follow us on Twitter for more articles about this topic.
Source: Tsinghua University Press.



