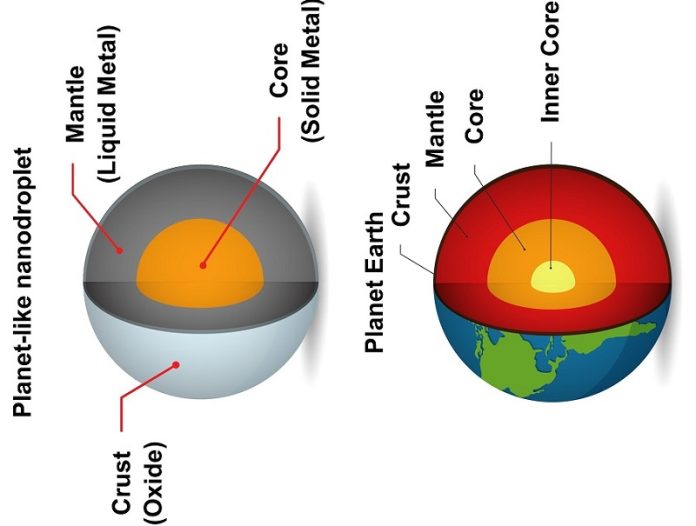
Scientists at RMIT University in Australia have managed to create a unique new form of liquid metal nanodroplets that resemble tiny planets.
Just like Earth, these minuscule droplets have an outer “crust,” a liquid metal “mantle,” and a solid “core.”
These tiny structures were developed to solve a problem researchers have been grappling with: how to evenly mix different types of metals on a nanoscale.
By using a high-temperature salt, the team managed to dissolve the metals fully in a liquid state, creating a consistent mixture in every droplet.
Why is this exciting?
Well, these liquid metal nanodroplets open up all sorts of possibilities for various fields such as flexible electronics, materials that change with temperature, catalysts, fuel cells, and even antimicrobial applications.
When scientists tried to create these tiny metal droplets in the past, they faced a challenge. They used a process called “sonication,” which uses sound waves to agitate the liquid metal, but the different metals in the mixture would separate out instead of staying mixed.
This happened because the process was done at relatively low temperatures, close to room temperature.
“Imagine trying to dissolve sugar in cold water versus warm water,” explains lead author Caiden Parker. “Just like sugar dissolves better in warm water, certain metals dissolve better in warmer liquid metal.”
In their new study, the RMIT researchers decided to crank up the heat— all the way to 400°C! This high temperature ensured that the target metal completely dissolved.
They also introduced a type of salt called sodium acetate, which remains stable at these temperatures.
The result was incredible: tiny nanodroplets that resembled mini planets. These “planets” had a solid outer shell, a liquid metal mantle, and a solid core.
The presence of this solid core is the secret behind the new technique’s success. It ensures the same amount of target metal gets “locked up” in each droplet, creating a consistent mixture.
The researchers also found that these planet-like nanodroplets were distributed evenly, making it even easier to work with them.
One potential application of these nanodroplets could be in speeding up chemical reactions, a process known as catalysis. For instance, the copper and gallium droplets they created showed promising results in speeding up the breakdown of ethanol, which could be useful in ethanol fuel cells.
Next, the researchers plan to test this technique using other combinations of metals. The great thing about liquid metal systems is that they can be adjusted for specific applications.
For instance, copper is a good electrical conductor, making it ideal for flexible electronics. Silver, on the other hand, is known for its antimicrobial properties, which could lead to exciting new medical applications.
These tiny, planet-like structures are like mini laboratories that allow scientists to study how molten metals behave on an atomic level. As Parker put it, “The potential applications of the new technology are extremely wide.
Any industries in need of nanomaterials can utilize the system, with the metals varying according to the application.” In short, it’s a small step for nanodroplets, but a giant leap for materials science!
Follow us on Twitter for more articles about this topic.
Source: FLEET.



