Researchers have made an exciting breakthrough in battery technology by developing a hybrid cell that not only stores and provides electricity but also produces valuable chemicals.
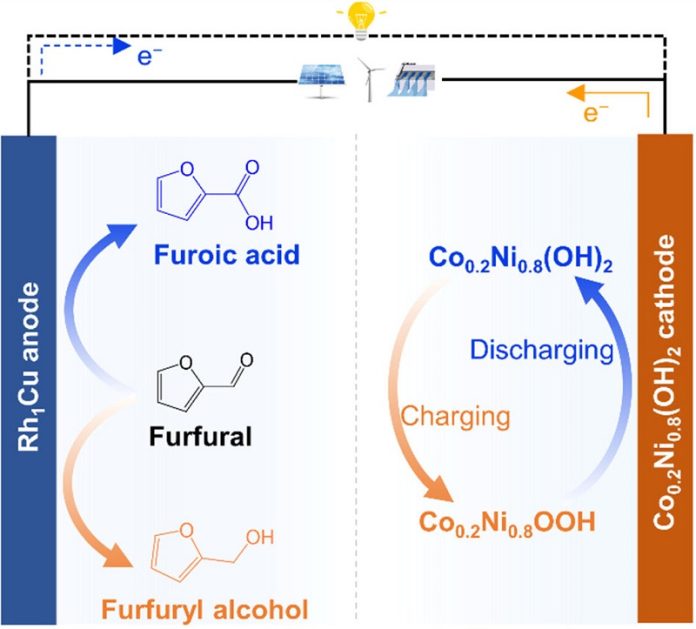
This innovative battery system combines the principles of rechargeable batteries and redox flow batteries, enabling the conversion of biomass-derived furfural into furfuryl alcohol or furoic acid during its operation.
The ability to generate value-added chemicals while storing energy enhances the cost efficiency of the battery system, marking a significant step towards more sustainable and economical rechargeable batteries.
Traditional rechargeable batteries store electricity in their electrodes, while redox flow batteries store electricity in chemicals stored in tanks.
The researchers aimed to combine these concepts to explore the potential of producing additional chemicals while storing or providing energy.
Their focus was on developing a bifunctional metal catalyst for the anode and identifying an appropriate cathode material.
The team utilized a rhodium-copper single-atom alloy as a bifunctional catalyst for the anode.
This catalyst facilitated the conversion of furfural, present in the battery’s electrolyte, into furfuryl alcohol during the charging process and furoic acid during discharge.
For the cathode, they identified a cobalt-doped nickel hydroxide material, similar to those used in conventional nickel-zinc or nickel-metal hydride batteries.
The combination of the bifunctional catalyst and cathode material resulted in a true dual-use battery system.
When charged, the hybrid batteries, connected in series, could power various devices like LED lights and smartphones. Simultaneously, the battery system continually produced furfuryl alcohol and furoic acid through a flow system, which conducted these valuable chemicals away from the battery.
The hybrid battery exhibited energy density and power density comparable to many common battery types.
While storing 1 kWh of energy, the system produced 0.7 kg of furfuryl alcohol. Conversely, when providing 0.5 kWh of power, equivalent to running a refrigerator for a couple of hours, 1 kg of furoic acid was produced.
It’s important to note that furfural is continuously fed into the system, and the separation of products from the electrolyte is necessary.
This hybrid battery concept represents a significant advancement in improving the sustainability and cost-effectiveness of rechargeable batteries.
However, further research and development are required to refine and enhance the concept for practical implementation.
The development of a hybrid battery capable of simultaneously storing electricity and producing valuable chemicals marks an exciting advancement in battery technology.
This innovative system holds promise for achieving more sustainable and economically efficient rechargeable batteries.
By generating value-added chemicals during the battery’s operation, this breakthrough paves the way for a greener future in energy storage.
The study was published in the journal Angewandte Chemie International Edition.



