There are many types of rocket fuel.
Some are more useful on a particular planet. And some can be created by bacteria.
A team from Georgia Tech has found a rocket fuel with an interesting mix of those characteristics that might be a focal point of in-situ resource utilization – on Mars.
2,3-butanediol might not be a household name like methane, which is commonly used as rocket fuel on Earth.
It’s primarily used in the manufacture of rubber products. But it does pack quite a punch when burned with liquid oxygen (LOX). Enough of a punch to be able to lift a spaceship into orbit on the Red Planet.
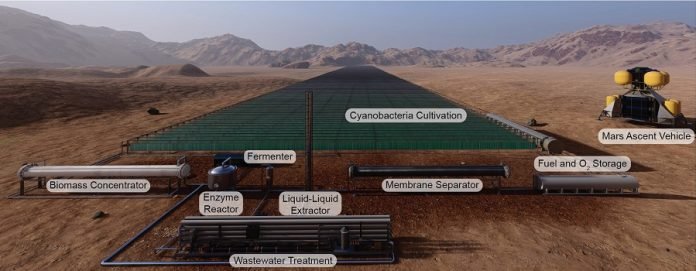
What’s more, 2,3-butanediol is relatively easy to synthesize. All that’s needed are some bioreactors and some CO2.
The bioreactors, which are the size of four football fields in the proposal described by the GT team, hold a multistage process that starts with cyanobacteria (also known as algae).
Cyanobacteria would photosynthesize as part of their natural life cycle, absorbing CO2 out of the Martian air.
After the algae does its work, it is introduced to enzymes that break it down into complex sugar molecules.
Those sugars are then fed to another bacteria – E. coli, a favorite of biology students everywhere.
Feasting on the sugars of the dead cyanobacteria, E. coli produces 2,3-butanediol, a type of rocket fuel, which must then be separated from the general soup of the bioreactor.
At the end of the process, there’s a propellant (2,3-butanediol) that can react with an oxidizer. Fortunately, this process also creates pure oxygen as a byproduct of one of the steps.
So a single process can pull CO2 from Mars’ atmosphere and make both the oxidizer and propellant needed for rocket fuel.
Traditional rocket propellant, such as methane, must be shipped from Earth if it is to be used in remote locales like Mars.
Costs for those shipments could reach billions of dollars for the amount of rocket fuel required to lift a human-loaded ship back on the way to Mars.
So why hadn’t 2,3-butanediol been offered up as a solution to this expensive rocket fuel creation problem previously?
Because it isn’t particularly good – on the Earth at least. Mars has a much smaller gravity well, making it possible for fuels with less power behind them to launch a rocket into orbit effectively. In addition, the lack of oxygen in the atmosphere makes 2,3-butanediol a much more attractive choice for propellant.
While this sounds like an idealized process of creating a valuable resource out of literally thin air on another planet, some hiccups must be overcome first. The first is whether the cyanobacteria and E. coli can live in the Martian environment.
Its harsh atmosphere and constant radiation bath can quickly debilitate living organisms, and it would likely be prohibitively expensive to enclose the rocket fuel farm in an environmental dome. So for the process to work, these hardy bacteria will have to operate in the open Martian atmosphere.
There are simulation chambers where this work could be done before a fully-fledged mission to Mars would be planned.
And that is exactly what the GT team hopes to do soon – test their process in a more realistic model of the arid Martian climate. With luck, they will help to introduce a new kind of organically grown rocket fuel to the mix on Mars.
Written by Andy Tomaswick.
Source: Universe Today.



