If there is one myth that has persisted through the years without much evidence, it’s this:
Multiply your dog’s age by seven to calculate how old they are in “human years.”
In other words, the old adage says, a four-year-old dog is similar in physiological age to a 28-year-old person.
But a new study by researchers at University of California San Diego School of Medicine throws that out the window.
Instead, they created a formula that more accurately compares the ages of humans and dogs.
The formula is based on the changing patterns of methyl groups in dog and human genomes — how many of these chemical tags and where they’re located — as they age.
Since the two species don’t age at the same rate over their lifespans, it turns out it’s not a perfectly linear comparison, as the 1:7 years rule-of-thumb would suggest.
The new methylation-based formula, published July 2 in Cell Systems, is the first that is transferrable across species.
More than just a parlor trick, the researchers say it may provide a useful tool for veterinarians, and for evaluating anti-aging interventions.
“There are a lot of anti-aging products out there these days — with wildly varying degrees of scientific support,” said senior author Trey Ideker, PhD, professor at UC San Diego School of Medicine and Moores Cancer Center.
“But how do you know if a product will truly extend your life without waiting 40 years or so?
What if you could instead measure your age-associated methylation patterns before, during and after the intervention to see if it’s doing anything?”
Ideker led the study with first author Tina Wang, PhD, who was a graduate student in Ideker’s lab at the time.
The formula provides a new “epigenetic clock,” a method for determining the age of a cell, tissue or organism based on a readout of its epigenetics — chemical modifications like methylation, which influence which genes are “off” or “on” without altering the inherited genetic sequence itself.
Epigenetic changes provide scientists with clues to a genome’s age, Ideker said — much like wrinkles on a person’s face provide clues to their age.
Ideker and others have previously published epigenetic clocks for humans, but they are limited in that they may only be accurate for the specific individuals on whom the formulas were developed. They don’t translate to other species, perhaps not even to other people.
Ideker said it was Wang who first brought the dog idea to him.
“We always look at humans, but humans are kind of boring,” he said. “So she convinced me we should study dog aging in a comparative way.”
To do that, Ideker and Wang collaborated with dog genetics experts Danika Bannasch, DVM, PhD, professor of population health and reproduction at UC Davis School of Veterinary Medicine, and Elaine Ostrander, PhD, chief of the Cancer Genetics and Comparative Genomics Branch at the National Human Genome Research Institute, part of the National Institutes of Health.
Bannasch provided blood samples from 105 Labrador retrievers. As the first to sequence the dog genome, Ostrander provided valuable input on analyzing it.
Dogs are an interesting animal to study, Ideker said. Given how closely they live with us, perhaps more than any other animal, a dog’s environmental and chemical exposures are very similar to humans, and they receive nearly the same levels of health care.
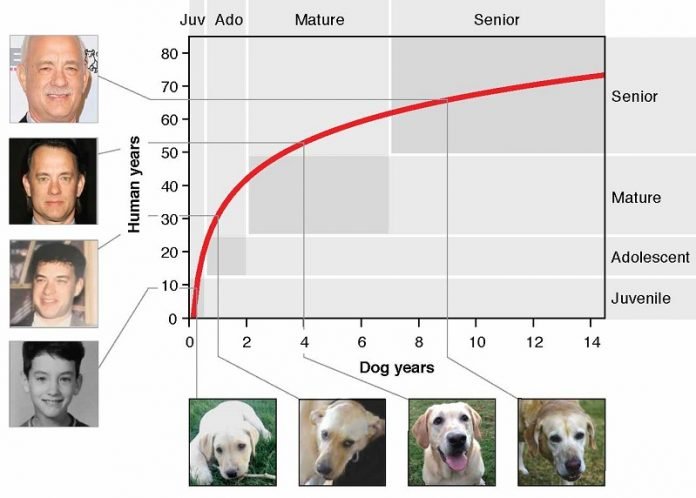
It’s also important that we better understand their aging process, he said, as veterinarians frequently use the old 1:7 years ratio to determine a dog’s age and use that information to guide diagnostic and treatment decisions.
What emerged from the study is a graph that can be used to match up the age of your dog with the comparable human age. The comparison is not a 1:7 ratio over time. Especially when dogs are young, they age rapidly compared to humans. A one-year-old dog is similar to a 30-year-old human. A four-year-old dog is similar to a 52-year-old human. Then by seven years old, dog aging slows.
“This makes sense when you think about it — after all, a nine-month-old dog can have puppies, so we already knew that the 1:7 ratio wasn’t an accurate measure of age,” Ideker said.
According to Ideker, one limitation of the new epigenetic clock is that it was developed using a single breed of dog, and some dog breeds are known to live longer than others. More research will be needed, but since it’s accurate for humans and mice as well as Labrador retrievers, he predicts the clock will apply to all dog breeds.
Next, the researchers plan to test other dog breeds, determine if the results hold up using saliva samples, and test mouse models to see what happens to their epigenetic markers when you try to prolong their lives with a variety of interventions.
Meanwhile, Ideker, like many other dog owners, is looking at his own canine companion a little differently now.
“I have a six-year-old dog — she still runs with me, but I’m now realizing that she’s not as ‘young’ as I thought she was,” Ideker said.



