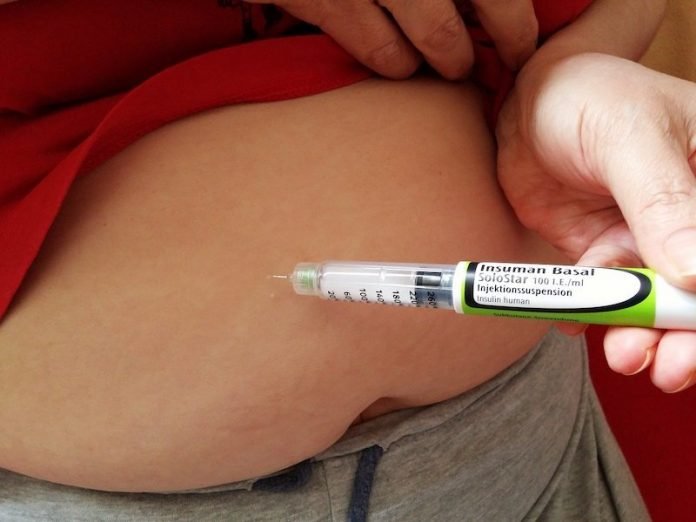
In a new study, researchers found that adults with type 2 diabetes requiring insulin therapy can safely achieve good blood sugar control using regular human insulin (RHI) in a wearable, patch-like insulin delivery device called V-Go®.
The results of the study suggest “a more affordable option” for insulin therapy than a newer insulin type.
The research was conducted by a team at the Dallas Diabetes Research Center.
The modern insulins—rapid-acting insulin (RAI) analogs—have dominated the mealtime insulin market for years, but skyrocketing prices have resulted in concerns of affordability and whether their differences from other available insulins are clinically relevant.
When injected by an insulin pen or insulin syringe, RHI—an older and less expensive insulin—takes longer to reach the bloodstream and has a longer duration of action compared to modern RAI. These differences can influence blood glucose control.
V-Go is a 24-hour small, disposable mechanical device that, according to its manufacturer, Valeritas, Inc., is cleared for use with RAI in adults with diabetes, is easy to use and worn like a patch on the skin.
It has the ability to deliver both a steady continuous subcutaneous infusion of insulin for 24 hours and mealtime insulin dosing on demand.
The study was conducted at three study centers in the southern United States and evaluated the safety and effectiveness of delivering RHI with V-Go in 113 adults with type 2 diabetes who were currently using the device filled with RAI.
The team assigned 54 patients to continue using the V-Go with RAI, and they assigned another 59 patients to switch the insulin used to fill V-Go from RAI to RHI.
Over the 14-week study, which Valeritas, Inc. supported with an educational grant, the researchers measured the between group difference in the average change in hemoglobin A1c, a measure of long-term blood glucose control.
The research team reported that improvements in A1c were observed, with a decrease of 0.6% in the RHI group and a decrease of 0.38% in the RAI group.
The study met its primary endpoint of noninferiority, or similar blood glucose control.
The researchers found no between-group differences in the total daily dose of insulin required or in episodes of low blood sugar, a measure of safety.
By continuously infusing insulin for 24 hours with V-Go, differences in duration of insulin action are minimized between the two insulins.
Our results support that RHI can be safely and effectively used when delivered by V-Go.
The lead author of the study is Pablo Mora, M.D., an endocrinologist at Dallas Diabetes Research Center at Medical City, Dallas, Texas.
The study is published in the Journal of the Endocrine Society.
Copyright © 2020 Knowridge Science Report. All rights reserved.



