The batteries that power our phones, laptops, and electric cars are getting better every year, but the materials inside them may soon need an upgrade.
A team of international researchers, led by Yan Yao, professor at the University of Houston’s Cullen College of Engineering, has taken a close look at alternative metals that could make batteries last longer and charge faster.
Their review, published in Science, highlights both the opportunities and challenges of moving beyond today’s lithium-ion technology.
Right now, graphite is the standard material used for the anode—the part of the battery that stores and releases energy. But graphite is reaching its limits.
Lithium metal, one possible replacement, offers about ten times more charge storage than graphite. Unfortunately, lithium has a serious drawback: as it cycles, it tends to form tiny needle-like structures called dendrites. These dendrites can pierce through the battery, causing dangerous short circuits.
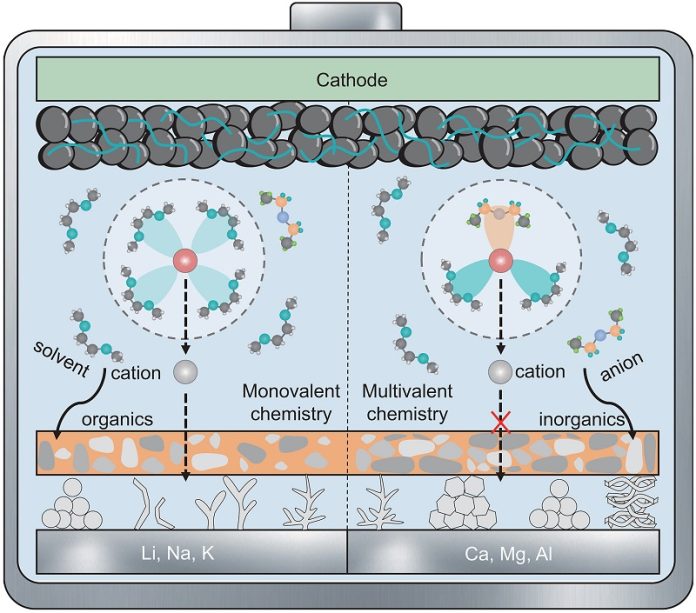
That’s why scientists are also exploring multivalent metals like magnesium, calcium, and aluminum. These metals carry more than one charge per ion, which means they could store more energy in a smaller space and do so at a lower cost.
They are also more abundant and generally safer. The downside, however, is that their ions move more slowly, which can make charging sluggish. The trade-off is that they are far less likely to form dendrites, offering greater stability.
To make these new battery designs work, researchers are developing creative solutions. One approach is to create textured anode surfaces that guide the metals to deposit smoothly, avoiding harmful clumping. Another strategy is designing new electrolytes—the liquids that carry ions between the anode and cathode—that can help ions move more efficiently and create protective films that extend the battery’s life.
Yao explained that this area of research is still in its early stages, but progress is accelerating. “This work underscores the need for continued research to overcome the technical barriers of multivalent metal batteries,” he said. “Advances in electrode design, electrolyte chemistry, and battery architecture are crucial to harness the full potential of these materials.”
The review also offers new design principles for different types of batteries. For example, lithium and other monovalent systems may work best with electrolytes that are weakly binding, while multivalent systems may require electrolytes that are strongly binding but avoid ion pairing. These insights provide a roadmap for how future batteries might be built.
Collaborators from Singapore’s Institute of Materials Research and Engineering, Zhejiang University in China, and Seoul National University in South Korea contributed to the review, highlighting the global interest in finding better alternatives.
With demand for sustainable and high-performance energy storage growing worldwide, this research could help guide the development of next-generation batteries.
If successful, the future may bring electric vehicles that drive farther, smartphones that last longer, and laptops that charge in a fraction of the time—all powered by new metals beyond lithium.
Source: University of Houston.



