From smartphones to electric cars, lithium-ion batteries power much of our modern world.
But while they are essential for clean energy and portable power, they come with big challenges.
Most lithium-ion batteries use flammable organic solvents, which make them a fire risk, require energy-intensive manufacturing, and are difficult to recycle.
These problems raise costs, create safety concerns, and harm the environment—driving the search for safer, cleaner alternatives.
Researchers at the Institute of Science Tokyo believe they have found one. Led by Specially Appointed Professor Yosuke Shiratori and Associate Professor Shintaro Yasui, the team has developed a new quasi-solid electrolyte called 3D-Slime Interface Quasi-Solid Electrolyte, or 3D-SLISE.
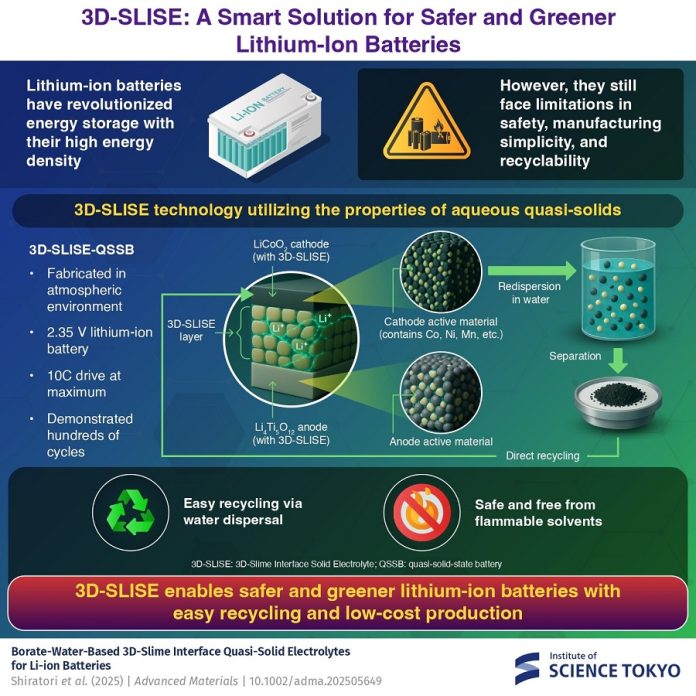
This soft, slime-like material allows lithium-ion batteries to be made safely under normal room conditions—without dry rooms, glove boxes, or high-temperature ovens. Even better, it makes recycling simple, so valuable materials can be recovered without harsh chemicals.
The team’s work, published in Advanced Materials on July 9, 2025, shows how 3D-SLISE could transform battery manufacturing.
The electrolyte is created by blending amorphous lithium tetraborate with lithium salt (LiFSI), carboxymethyl cellulose, and water. The result is a gel-like matrix that enables lithium ions to move freely in all directions, which is essential for efficient charging and discharging.
Depending on how it’s used, 3D-SLISE comes in two types. Type E is mixed with electrode materials to form the cathode and anode layers, while Type S is used as the quasi-solid electrolyte layer sandwiched between them.
The materials are simply dried at room temperature, making the process ideal for industrial-scale production without the high energy costs of traditional methods.
When tested in a 2.35-volt lithium-ion battery, 3D-SLISE performed impressively. Under a 3C rate—meaning the battery could be charged or discharged in just 20 minutes—it delivered over 400 charge/discharge cycles in normal room conditions.
It also showed high ionic conductivity (2.5 milli-siemens per centimeter) and low activation energy (0.25 electron volts), indicating that it works efficiently at everyday temperatures.
But performance isn’t the only advantage. Because 3D-SLISE is water-based and contains no traditional binders like polyvinylidene di-fluoride (PVDF), recycling becomes much simpler. Used batteries can be taken apart, and the electrodes can be soaked in water to release valuable active materials such as cobalt.
This direct recovery method avoids the need for energy-heavy or toxic recycling processes, making it both cost-effective and environmentally friendly.
“Using this technology, it is possible to directly reclaim valuable elements like cobalt, contributing to a more sustainable and reliable supply of critical battery materials,” said Yasui.
The researchers see 3D-SLISE as a major step toward a circular battery economy, where batteries are not just used and discarded, but reused and recycled efficiently. With its combination of safety, recyclability, and low-impact production, it could find applications in everything from consumer electronics to large-scale energy storage systems.
If widely adopted, this “slimy” innovation could lower manufacturing costs, reduce environmental harm, and make lithium-ion batteries safer—helping to power a cleaner, more sustainable future.



