Right after the Big Bang, about 13.8 billion years ago, the universe was a hot, dense sea of energy and particles.
But in just a few seconds, it cooled down enough for the first elements to form—mainly hydrogen and helium. These elements remained in a charged, or ionized, state for quite some time.
It wasn’t until about 380,000 years later that the universe had cooled enough for electrons to combine with these nuclei to form neutral atoms.
This period, known as “recombination,” allowed light to travel freely through space and set the stage for chemistry to begin.
The first molecule to form in the universe was helium hydride (HeH⁺), made when a neutral helium atom bonded with a positively charged hydrogen nucleus.
Although simple, this molecule marked the beginning of the first chemical reactions in the cosmos.
It also played a crucial role in forming molecular hydrogen (H₂), which is the most common molecule in the universe today.
After recombination, the universe entered what scientists call the “dark ages.” There were no stars or galaxies yet—only cold, dark gas floating through space.
Several hundred million years passed before the first stars finally lit up the universe. But even during this early time, molecules like HeH⁺ and H₂ were quietly doing important work.
To form stars, clouds of gas must collapse under gravity. But as gas compresses, it heats up—and unless that heat can escape, the collapse will stop.
Molecules help solve this problem. When they collide with other particles, they can absorb energy and release it as light, cooling the gas. This cooling is essential for the birth of stars.
However, hydrogen atoms on their own aren’t very good at this cooling process below 10,000°C. Molecules like HeH⁺, which have a strong dipole moment (a property that helps them radiate energy more effectively), can keep cooling the gas even at much lower temperatures.
This made HeH⁺ a potentially key player in early star formation.
But there’s a catch—HeH⁺ is not stable forever. It reacts easily with hydrogen atoms, breaking apart into a helium atom and a new ion called H₂⁺. This ion then reacts with another hydrogen atom to form molecular hydrogen (H₂), which is also crucial for cooling gas in space.
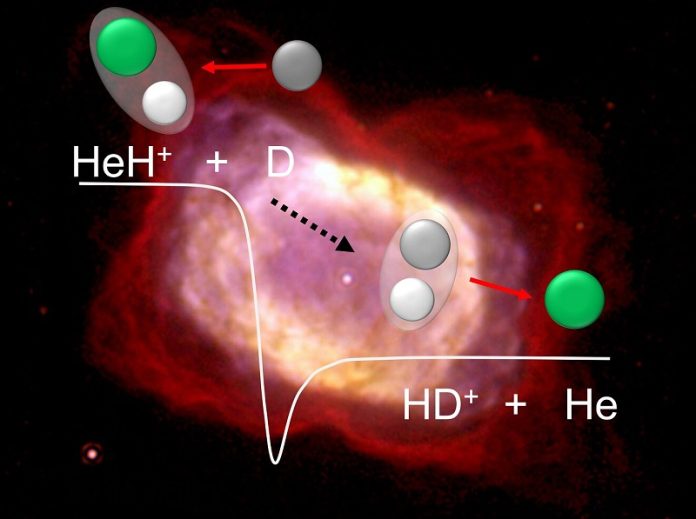
To better understand this, scientists at the Max Planck Institute for Nuclear Physics in Heidelberg recreated one of these early-universe reactions in the lab.
Using a special device called the Cryogenic Storage Ring, they studied what happens when HeH⁺ reacts with deuterium, a heavy form of hydrogen.
The results were surprising: unlike earlier theories, the reaction didn’t slow down at lower temperatures. In fact, it remained steady, suggesting these reactions were far more important in the early universe than scientists once thought.
New theoretical work also helped explain this finding. A group of researchers discovered an error in previous calculations and, after correcting it, their predictions matched the experiment perfectly.
This research helps us better understand how the first stars were born. It shows that the simple chemistry of the early universe—starting with HeH⁺—was essential in shaping the cosmos we see today.



