A team of scientists led by Georgia Tech has made a groundbreaking discovery that could change the way we use and extract rare earth elements—materials essential for modern technologies like smartphones, electric vehicles, medical devices, and military equipment.
They’ve identified a new chemical state for praseodymium, a rare earth metal, which could open the door to new technological advances and more efficient mining methods.
Rare earth elements, also known as lanthanides, are a group of metals that are notoriously difficult to separate from each other.
They all look and behave very similarly, making the process of isolating them slow, expensive, and wasteful. Yet, they are in high demand because of their unique magnetic and optical properties.
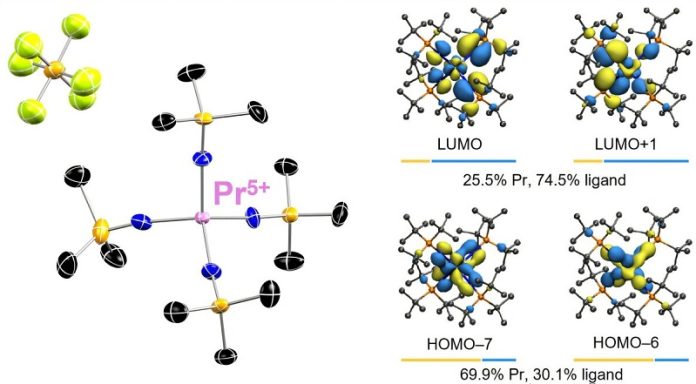
Now, researchers have observed praseodymium in a state never seen before—a 5+ oxidation state.
Oxidation is a process where an element loses electrons, often when it reacts with oxygen. Everyday examples include an apple turning brown or metal rusting.
Most rare earth elements, including praseodymium, are only stable in a 3+ oxidation state under normal conditions. This discovery of a 5+ state is a major leap forward.
According to Henry “Pete” La Pierre, associate professor at Georgia Tech and lead author of the study, this discovery is like uncovering a whole new dimension in the periodic table.
“It gives us ideas for where to go next,” he said. “Each oxidation state gives the element different chemical and physical properties, so finding a new one opens up new possibilities.”
This achievement was long suspected—scientists as far back as the 1890s believed lanthanides could have a 5+ oxidation state, but no one had ever been able to confirm it until now. La Pierre and his colleagues at the University of Iowa and Washington State University finally made it possible.
Why does this matter? Currently, we only use lanthanides in their 3+ oxidation state for commercial applications. If we can stabilize and control higher oxidation states like 5+, we might unlock entirely new magnetic and optical behaviors. This could lead to better electronic devices, new quantum technologies, and more efficient ways to recycle or separate rare earth metals.
Improving how we separate these metals is crucial. Rare earth elements are often found mixed together in nature, and separating them is a tedious process that produces a lot of waste.
With global demand rising and supply chains under strain, especially in the U.S., this discovery could help make rare earth mining and recycling cleaner, cheaper, and more sustainable.
The study, titled “Praseodymium in the Formal +5 Oxidation State”, was published in Nature Chemistry and marks a major step forward in rare earth research.



