Scientists have found a new way to make better batteries using a common lithium salt, and it could change the way batteries are manufactured.
The key lies in a process called sublimation, where a solid turns directly into a gas without becoming a liquid first.
It’s the same process that gives a comet its glowing tail when it gets close to the sun.
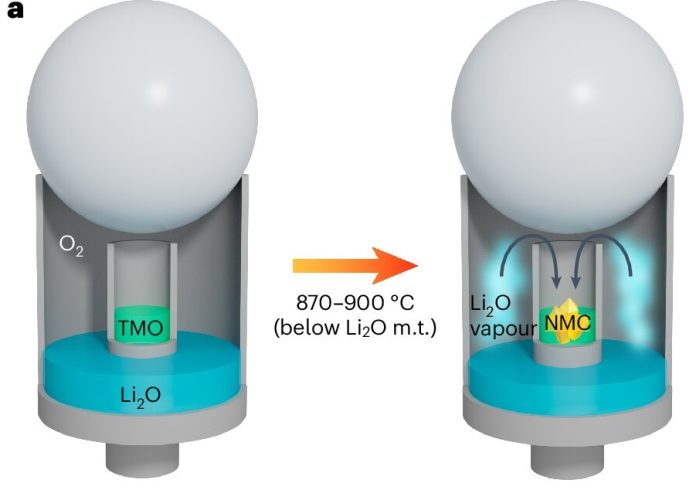
In this new study, researchers at the U.S. Department of Energy’s Pacific Northwest National Laboratory (PNNL) discovered that lithium oxide (Li₂O), when heated, gives off vapors that help form single-crystal materials—an ideal structure for making strong, long-lasting batteries.
Their findings, published in Nature Energy, could lead to cheaper and more efficient battery production.
Right now, most lithium-ion batteries are made using nickel, cobalt, and manganese.
Adding more nickel improves battery performance and cuts down on cost, but there’s a downside. Nickel-rich materials tend to form clumps, or “polycrystals,” which are like cookies full of chocolate chips.
These clumps develop weak spots over time as the battery charges and discharges, leading to cracks that reduce battery life.
To solve this problem, scientists want to create “single crystals”—materials with a uniform structure and no weak boundaries.
This would be like baking a cookie where the chocolate is spread evenly throughout, making it more stable and longer-lasting.
Researchers have been trying to find better ways to make these single crystals. Typically, lithium hydroxide (LiOH) is used in production because it melts at a lower temperature. Li₂O, on the other hand, melts at a very high temperature (1,438°C), so it’s been mostly ignored—until now.
When the PNNL team heated Li₂O to around 900°C and combined it with nickel-rich materials, they were surprised to see single crystals form easily.
After many tests and help from Thermo Fisher Scientific’s advanced microscopes, they confirmed that the effect was caused by sublimation. The Li₂O vapor seeps into the other materials and triggers the formation of strong, single-crystal structures.
The team also discovered something even more promising: they could use this process to recycle old battery materials. By simply mixing and heating waste polycrystal material with Li₂O, they turned it into high-quality single crystals.
These new crystals performed well even after 1,000 charge-discharge cycles, showing excellent long-term stability.
This breakthrough could significantly reduce manufacturing costs and improve battery lifespan. However, there are still some hurdles. Li₂O isn’t widely used in industry yet, and its current price is high. But it can be made from other common lithium salts like LiOH, which may help bring down costs.
The PNNL team, led by battery expert Dr. Jie Xiao, is now working with industry partners to scale up the process. They hope to start supplying single-crystal battery materials to manufacturers by 2026. If successful, this new method could help power everything from smartphones to electric cars more reliably and affordably.



