The batteries in our phones, devices, and electric cars rely on metals like lithium and cobalt. Mining these materials is energy-intensive and harmful to the environment.
As we move toward a greener future, finding alternatives to metal-based batteries is essential.
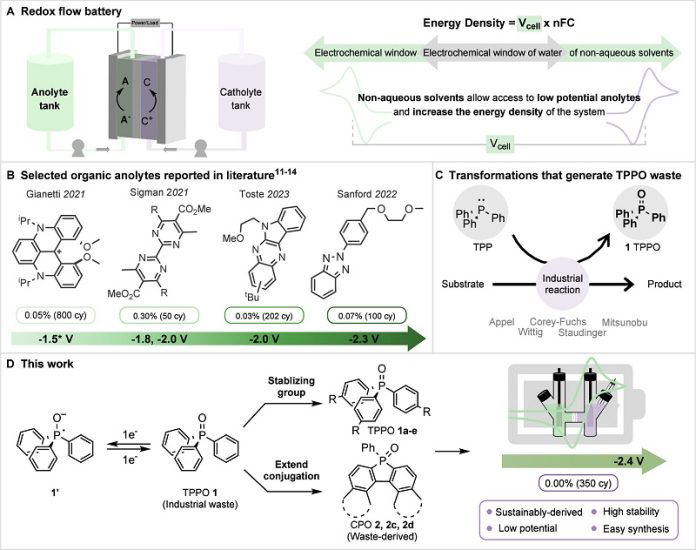
Now, researchers at Northwestern University have made a major breakthrough by transforming an industrial waste product into a new kind of energy storage for batteries.
This innovative approach could pave the way for sustainable, large-scale energy storage.
The waste product, called triphenylphosphine oxide (TPPO), is a byproduct of many industrial processes, such as the production of vitamins. While thousands of tons of TPPO are made every year, it has no use and must be carefully discarded.
In their study, published in the Journal of the American Chemical Society, the team used a simple “one-pot” chemical reaction to turn TPPO into a molecule capable of storing energy.
This development opens the door for waste-derived organic redox flow batteries, which are well-suited for storing renewable energy at grid scale.
“Battery research has been dominated by engineers and materials scientists,” said lead researcher Christian Malapit. “We’re showing how synthetic chemists can transform waste into valuable energy solutions. This discovery highlights the potential of turning trash into treasure for green energy.”
Redox flow batteries work differently from the lithium-ion batteries in most devices today. Instead of storing energy in solid electrodes, they store energy in liquid electrolytes that use chemical reactions to transfer energy back and forth. These batteries are especially promising for large-scale applications, like storing energy from solar and wind power. While not as energy-efficient as lithium-ion batteries, redox flow batteries are more stable and better for long-term, high-capacity use.
The Northwestern team achieved a key milestone by creating a TPPO-based molecule that offers both high energy density and long-lasting stability. After running over 350 charge-discharge cycles, the battery showed almost no loss in capacity. This is a major step forward since combining energy density and stability has been a challenge for organic molecules in battery research.
“This is the first time phosphine oxides have been used as the active component in batteries,” said Malapit. “By addressing their instability, we’ve opened up new possibilities for energy storage.”
The team hopes other researchers will build on their work, further optimizing TPPO for use in redox flow batteries. If successful, this technology could reduce dependence on mined materials and create a greener, more sustainable future for energy storage.



