Lithium is an essential material for making batteries that power electric cars, smartphones, and other electronic devices.
However, traditional ways of extracting lithium from the earth use a lot of energy and cause environmental damage.
Now, researchers at Penn State University have developed a new method that could make lithium extraction cheaper, cleaner, and more efficient.
Their process, which uses electric current and hydrogen peroxide, could reduce the cost of lithium extraction by 35.6% and lower carbon emissions by 75.3% compared to traditional methods.
The team, led by Professor Feifei Shi, published their findings in the journal Nature Communications.
Today, lithium is mainly extracted in two ways:
- Brine ponds – Large pools of saltwater are left to evaporate over several months, leaving behind lithium-rich salt. This process is slow, requires huge amounts of land, and damages ecosystems by turning fertile soil into barren land.
- Ore mining – Lithium can also be mined from spodumene, a common mineral found in rocks. However, extracting lithium from this ore requires very high temperatures (up to 1,100°C) and strong acids. This process is expensive, energy-intensive, and potentially dangerous for workers.
Both methods have significant environmental and economic drawbacks, pushing scientists to find a more sustainable alternative.
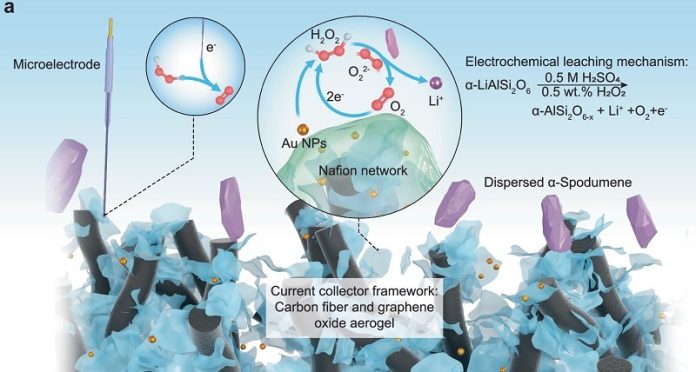
Instead of using extreme heat and harsh chemicals, the Penn State team has developed a way to extract lithium using an electric field and a mild chemical process.
This method, called electrochemical leaching, works by applying electricity to the mineral, which frees lithium ions and dissolves them into a liquid form.
At first, the researchers found that the electric current alone wasn’t strong enough to extract large amounts of lithium. To fix this, they added hydrogen peroxide, which helps speed up the reaction and improve efficiency.
✔ Lower Costs – The process requires less energy and fewer chemicals, reducing production costs by 35.6%.
✔ Faster Extraction – Unlike brine ponds that take months, this method instantly releases lithium when electricity is applied.
✔ Eco-Friendly – It cuts carbon emissions by 75.3%, since it relies on electricity rather than fossil fuels.
✔ Safer for Workers – No need for extreme heat or hazardous acids, making it safer for people working in lithium extraction.
Although this breakthrough is promising, more research is needed before it can be used on a large scale. The next goal is to develop a way to turn the extracted lithium into solid forms like lithium chloride or lithium hydroxide, which can be directly used by battery manufacturers.
Professor Zhen Lei, a co-author of the study, believes this method has huge commercial potential. “Our approach relies almost entirely on electricity, which makes it much more energy-efficient,” he said.
“If it works well for large-scale lithium extraction, it could significantly reduce the environmental impact of lithium mining.”
Professor Shi is also optimistic. “We believe this could be a revolution in lithium extraction,” he said. “Electrochemistry has the potential to transform how we mine and process minerals, making it more sustainable for the future.”
With the global demand for electric vehicles and renewable energy storage growing rapidly, finding a cleaner, faster, and more affordable way to extract lithium is more important than ever.
If this new electrochemical method proves successful on a large scale, it could transform the industry, making lithium production more sustainable and accessible.
Penn State’s research marks an exciting step toward a greener future, where we can power our devices and vehicles without harming the planet.



