Imagine a world where we produce clean hydrogen fuel using just sunlight and water.
This vision could become a reality thanks to breakthroughs by Japanese researchers who are developing new photocatalytic technology.
Their work is paving the way for large-scale hydrogen production without relying on fossil fuels.
Hydrogen fuel is a promising alternative energy source, but most of the hydrogen we use today comes from natural gas, which still contributes to carbon emissions.
Scientists led by Prof. Kazunari Domen and Dr. Takashi Hisatomi at Shinshu University are working on a cleaner solution: using sunlight to split water into hydrogen and oxygen.
Their recent progress, published in Frontiers in Science, highlights the challenges and potential of this revolutionary technology.
The process of splitting water into hydrogen and oxygen requires photocatalysts—materials that trigger chemical reactions when exposed to light. These reactions separate water molecules into their components.
There are two main types of photocatalytic systems:
- One-step systems, where a single photocatalyst splits water directly. These are simple but inefficient, converting only a tiny fraction of solar energy into hydrogen.
- Two-step systems, which use two different photocatalysts to separately produce hydrogen and oxygen. These are more efficient but still require further development to be practical.
One challenge is the efficiency of converting sunlight into hydrogen. The process needs to be more effective to make it cost-competitive with current methods, like extracting hydrogen from natural gas. Another issue is finding robust photocatalysts that can handle daily start-ups and shut-downs as the sun rises and sets.
Safety is another concern. Splitting water often produces oxyhydrogen—a mixture of hydrogen and oxygen—which is highly explosive.
The researchers have identified ways to manage this risk. For example, they found that igniting oxyhydrogen in small, narrow compartments reduces the risk of explosions. They also discovered that soft PVC plastic can limit destructive effects if ignition occurs.
In their research, Domen and Hisatomi’s team tested a 100-square-meter reactor over three years. Interestingly, the reactor performed even better in natural sunlight than in controlled lab settings, achieving solar-to-hydrogen conversion rates about 1.5 times higher. However, the current maximum efficiency is still just 1%, and the goal is to break the 5% barrier.
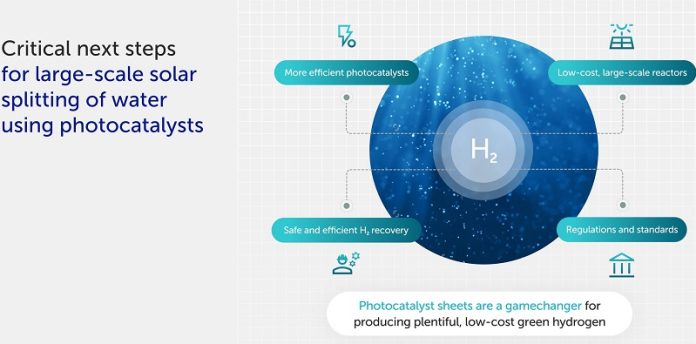
To make hydrogen fuel a viable alternative, researchers need to develop more efficient photocatalysts and scale up experiments with larger reactors.
This will also require collaboration to establish safety standards, efficiency benchmarks, and industry regulations. Accreditation and licensing processes will ensure safe and effective development of the technology.
“If we can improve solar-to-chemical conversion efficiency to practical levels, it will attract more researchers and investment,” says Prof. Domen. “This could accelerate advancements in mass production, gas separation, and infrastructure development.”
Hydrogen fuel derived from sunlight and water has the potential to revolutionize energy systems and significantly reduce reliance on fossil fuels.
With ongoing innovation, this clean and sustainable energy source could power a greener future.



