As the world shifts toward renewable energy, finding ways to store large amounts of electricity efficiently has become a major focus.
Lithium-ion batteries, while popular, aren’t ideal for every application, especially large-scale energy storage.
Now, researchers at the Technical University of Munich (TUM) have created a new method that could make zinc batteries a top choice for storing renewable energy, thanks to a breakthrough protective layer.
Zinc batteries are a promising alternative to lithium batteries because zinc is both cheaper and more widely available.
However, they have faced a big problem: they don’t last long enough. Without a protective layer, zinc batteries can fail after a few thousand charge and discharge cycles.
But this new innovation changes the game, as it could help zinc batteries last for hundreds of thousands of cycles.
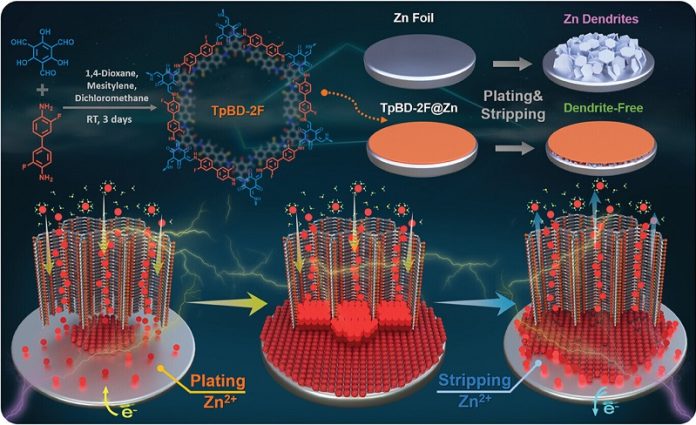
The secret lies in a special protective layer designed for the zinc anodes (the negatively charged part) of these batteries.
Over time, zinc anodes tend to grow “dendrites,” tiny needle-like structures that can short-circuit the battery.
Additionally, unwanted chemical reactions produce hydrogen gas, causing corrosion and other issues. These challenges have kept zinc batteries from becoming a go-to solution for large-scale energy storage.
The research team at TUM, led by Professor Roland A. Fischer, Chair of Inorganic and Metal-Organic Chemistry, developed a new solution to these problems.
They created a porous organic polymer layer called TpBD-2F, which forms a thin but strong film on the zinc anode.
This ultra-thin layer allows zinc ions to move easily through nano-sized channels, while keeping water away from the zinc anode, preventing corrosion and dendrite growth.
By controlling these reactions, the team has successfully extended the lifespan of zinc batteries by “several orders of magnitude,” potentially making them last hundreds of times longer.
Da Lei, a Ph.D. student and lead author of the study, explained that these improved zinc-ion batteries could one day replace lithium-ion batteries in large-scale storage systems for renewable energy sources like solar or wind power.
While lithium batteries work well for mobile devices and electric vehicles, they are more expensive and have greater environmental impacts, making them less suitable for massive energy storage projects.
“This is truly a spectacular result,” said Professor Fischer. “Our team has shown that Da Lei’s approach to improving zinc batteries works and is controllable.” Fischer also mentioned that they’ve already developed a prototype button cell based on this innovation and sees great potential for scaling it up to larger applications.
With this foundation laid, engineers can now work on creating larger and more powerful zinc batteries for practical use.
If this technology advances, zinc batteries could offer a safer, more affordable, and more sustainable solution for storing renewable energy on a large scale.
This exciting development brings us one step closer to a cleaner, greener energy future.



