Researchers from the Korea Advanced Institute of Science and Technology (KAIST) have developed a new system for producing hydrogen safely and efficiently.
The team, led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering, created a self-powered hydrogen production system based on a high-performance zinc-air battery.
Their findings were published in the journal Advanced Science.
Hydrogen is gaining attention as a clean fuel because it has a much higher energy density than fossil fuels like gasoline and diesel.
However, producing hydrogen often involves carbon dioxide (CO2) emissions, making it less environmentally friendly. Green hydrogen, which is produced by splitting water using renewable energy like solar or wind power, is a better option.
But this method has its own challenges due to the unstable nature of renewable energy sources, which can affect water-splitting efficiency.
To address these issues, researchers have looked into air cells, which can generate enough voltage for splitting water into hydrogen and oxygen.
However, air cells often rely on expensive metal catalysts, and the catalyst’s performance tends to degrade over time during repeated charging and discharging.
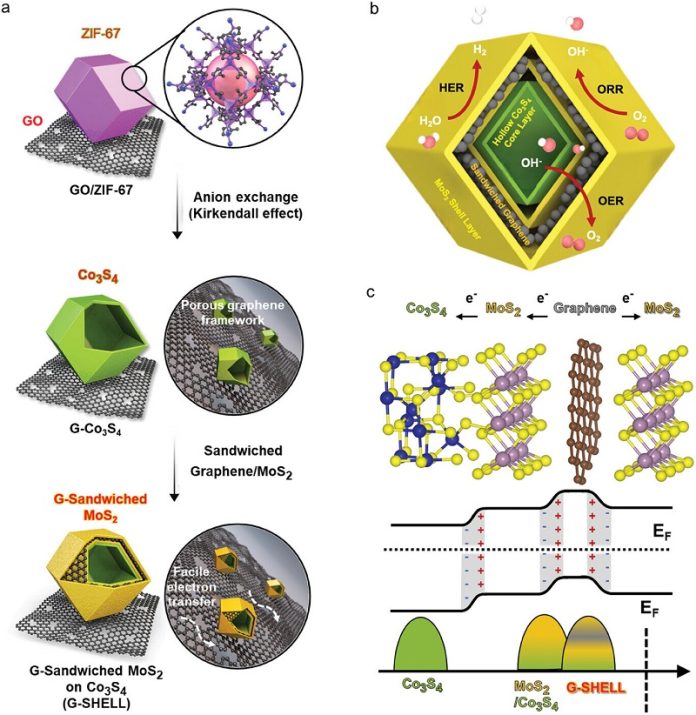
Professor Kang’s team tackled this problem by developing a new catalyst material called G-SHELL. This catalyst is effective for three key reactions needed in hydrogen production: oxygen generation, hydrogen generation, and oxygen reduction.
The team achieved this by growing nano-sized metal-organic frameworks on a material called graphene oxide. This approach allowed them to create a non-precious metal catalyst that performs well across all these reactions.
The researchers incorporated their G-SHELL catalyst into a zinc-air battery, which uses a water-soluble electrolyte. This battery has an impressive energy density of 797 Wh/kg—about five times higher than regular batteries. It also showed high output performance and remained stable even with repeated charging and discharging. Most importantly, the zinc-air battery eliminates the risk of fire, making it a safer choice for powering hydrogen production systems.
By connecting this zinc-air battery to a water-splitting system, the researchers created a self-powered hydrogen production system that can operate without the fluctuations in power that often occur with renewable energy sources. Professor Kang believes that their innovative system will be a significant step forward for green hydrogen production.
“The zinc-air battery-based hydrogen production system we developed is safe and simple to use,” said Professor Kang. “It is a new breakthrough that can overcome the limitations of current green hydrogen production.”
This new system offers an eco-friendly way to produce hydrogen, which could be key to future energy storage and clean fuel solutions. The zinc-air battery not only provides stable power but also reduces safety risks, making it a promising development in the push for sustainable energy.



