Researchers have finally solved a scientific puzzle that has stumped experts for more than half a century—the structure of aluminum oxide (Al2O3), also known as alumina.
Alumina is a highly insulating material used in everything from electronic components to ceramics.
Despite its wide use, scientists didn’t fully understand the arrangement of atoms on its surface, which is key to unlocking how chemical reactions happen, especially in catalytic processes.
Now, a team from TU Wien and the University of Vienna has cracked this mystery. Their findings, published in the journal Science, reveal the complex structure of alumina’s surface, ending a decades-long search for answers.
This discovery was one of the “three mysteries of surface science” identified back in 1997.
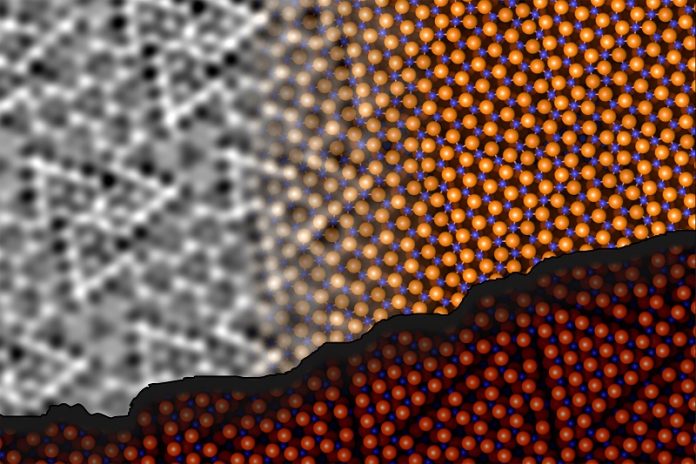
The researchers used a technique called noncontact atomic force microscopy (ncAFM) to investigate the surface of alumina.
This method allows scientists to get incredibly detailed images of the surface without physically touching it.
A sharp tip is positioned close to the material, and by measuring changes in frequency as the tip interacts with the surface atoms, scientists can map out the location of the atoms.
However, there’s a catch: this method doesn’t reveal what kind of atoms they are—whether they’re oxygen or aluminum. To solve this, the team attached a single oxygen atom to the tip of the microscope.
This oxygen atom is repelled by other oxygen atoms on the surface and attracted to aluminum atoms, allowing the researchers to figure out which atoms were which.
The team discovered that the surface of alumina doesn’t behave like the rest of the material inside the crystal.
The aluminum atoms on the surface actually move deeper into the material, bonding with oxygen atoms in lower layers.
This rearrangement helps stabilize the surface by reducing energy. Interestingly, although the structure changes, the ratio of aluminum to oxygen atoms stays the same—contrary to what scientists had previously believed.
Using advanced machine learning and computational methods, the team built a three-dimensional model of the alumina surface. This was no easy task, as the structure is highly complex. The use of cutting-edge technology allowed them to explore numerous possibilities and find the correct arrangement of atoms.
This breakthrough has major implications for several fields, including catalysis and material science.
By understanding how the surface of alumina is structured, scientists can now improve the design of materials that use it.
According to Jan Balajka, who led the research, this discovery opens up new opportunities for advancements in these fields and may even lead to innovations in how materials are designed for various applications.



