Rechargeable lithium-ion batteries are becoming more common in everything from smartphones and laptops to electric vehicles and energy storage systems.
However, the materials typically used in these batteries, like nickel and cobalt, are limited in supply, which makes them expensive and potentially unsustainable in the long run.
A new study led by researchers from the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) offers a promising alternative: manganese, a metal that is much more abundant and affordable.
Manganese is the fifth most abundant metal in the Earth’s crust and could be a game-changer for lithium-ion batteries, thanks to new findings on its use in a type of material called disordered rock salts, or DRX.
In previous research, DRX materials needed to be ground down to nanosized particles in an energy-intensive process to perform well as battery cathodes.
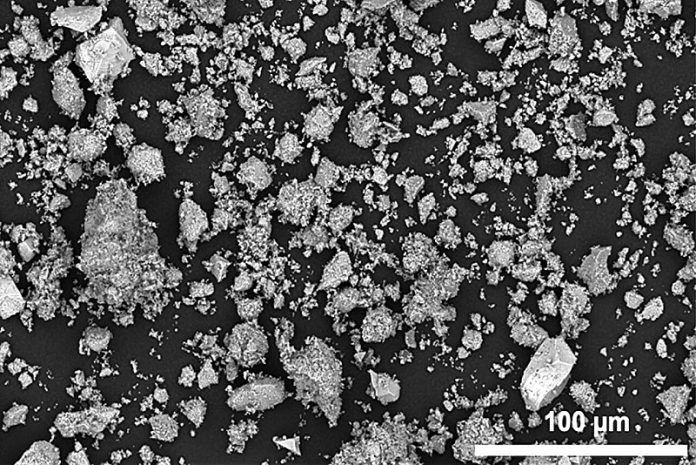
However, this new study found that manganese-based DRX cathodes can work effectively with much larger particles—about 1,000 times bigger than previously thought necessary.
This discovery could significantly reduce the energy and cost needed to produce these materials, making them more viable for widespread use.
The research was published on September 19 in the journal Nature Nanotechnology.
“There are many ways to generate power with renewable energy, but the key challenge is how to store it efficiently,” said Han-Ming Hau, a Ph.D. student at UC Berkeley and a researcher in battery technology at Berkeley Lab’s Ceder Group. “With our new approach, we can use a material that is abundant, low-cost, and easier to produce than many commercial battery cathode materials, while still storing as much energy and working just as well.”
The team developed a novel two-day process to prepare the manganese-based cathodes. This process involves first removing lithium ions from the cathode material and then heating it at a relatively low temperature of about 200 degrees Celsius. This method contrasts sharply with the existing process for manganese-based DRX materials, which can take over three weeks of treatment.
When viewed under an electron microscope, the manganese-based material revealed a unique nanoscale structure, characterized by special disordered areas known as “antiphase boundaries.” This structure, formed by the new processing method, actually improves battery performance, allowing the material to store and deliver energy more densely and efficiently.
The researchers also used X-ray techniques to study how the manganese material changes chemically during battery cycling. By understanding how the material behaves on both a microscopic and macroscopic level, the team hopes to develop new methods for making manganese-based cathodes and gain insights into how to engineer future battery materials.
“We now have a better grasp of the material’s unique nanostructure and a process to enhance its electrochemical performance,” Hau explained. “This is an important step toward making this material a practical option for real-world battery applications.”
This discovery brings us closer to more sustainable, efficient, and cost-effective lithium-ion batteries that could revolutionize energy storage in the future.
Source: Lawrence Berkeley National Laboratory.