Researchers at Griffith University have developed a new COVID-19 vaccine that can be administered through the nose, offering a promising alternative for those who are afraid of needles.
This next-generation vaccine, called CDO-7N-1, has shown strong potential in providing long-lasting immunity against COVID-19 and its variants with just a single dose.
The research, led by Professor Suresh Mahalingam from Griffith’s Institute for Glycomics, has been in progress for the past four years and was recently published in Nature Communications.
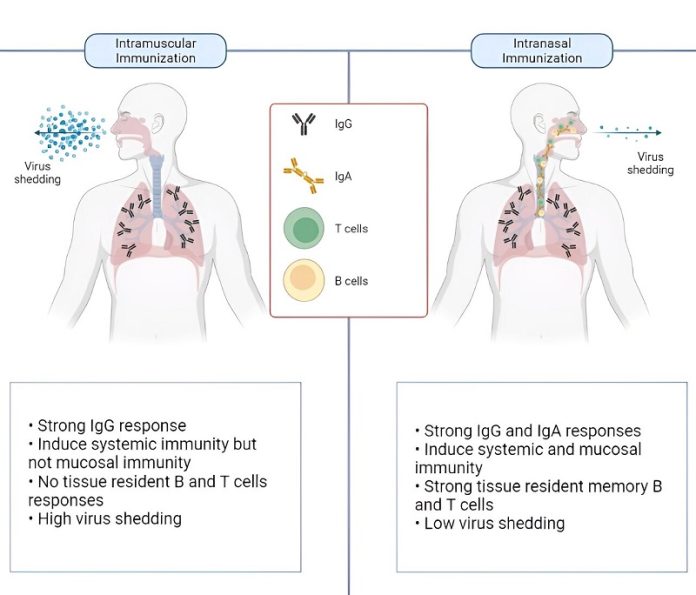
The vaccine is designed to be given intranasally, meaning it is sprayed into the nose rather than injected.
This method not only makes the vaccine easier to administer but also triggers immunity in both the nasal passages and the rest of the body.
Professor Mahalingam explained that CDO-7N-1 is a live-attenuated vaccine, which means it uses a weakened form of the virus to stimulate the immune system.
This type of vaccine offers several benefits, including strong and long-lasting immunity, often with just one dose.
“The vaccine induces strong memory responses in the nasal mucosa, offering long-term protection for up to a year or more,” he said.
One of the key advantages of CDO-7N-1 is its broad protection. Unlike mRNA vaccines, which target only the spike protein of the virus, this nasal vaccine induces immunity against all major SARS-CoV-2 proteins.
This makes it effective against all known variants of the virus, as well as SARS-CoV-1, the virus that caused the original SARS outbreak.
Dr. Xiang Liu, the lead author of the study, highlighted that the vaccine also provides strong protection against virus transmission and reinfection, helping to prevent the spread of COVID-19 and the emergence of new variants.
Another benefit is that the vaccine remains stable at 4°C (about 39°F) for up to seven months, making it particularly useful in low- and middle-income countries where cold storage is a challenge.
The vaccine has already been licensed to Indian Immunologicals Ltd, a leading vaccine manufacturer. Dr. K. Anand Kumar, Managing Director of the company, stated that they have completed all necessary studies and are preparing to move forward with clinical trials.
Professor Lee Smith, Acting Director of the Institute for Glycomics, expressed his excitement about the research findings, noting that this new vaccine could play a crucial role in making COVID-19 vaccination more accessible and effective worldwide.
The development of this needle-free, nasal vaccine represents a significant step forward in the fight against COVID-19, offering a more convenient and potentially more effective option for preventing the disease.
If you care about COVID, please read studies about vitamin D deficiency linked to severe COVID-19, death, and how diets could help manage post-COVID syndrome.
For more health information, please see recent studies about COVID infection and vaccination linked to heart disease, and results showing extracts from two wild plants can inhibit COVID-19 virus.
COVID-19 intranasal vaccine. Credit: Griffith University.



