One of the biggest challenges in the fight against climate change is figuring out how to store energy from renewable sources like wind and solar power.
Unlike fossil fuels, which store energy in their chemical bonds, renewable energy is more fleeting and harder to store efficiently.
Eric Detsi, an Associate Professor in Materials Science and Engineering, believes that the answer lies in better batteries.
However, the batteries we currently have aren’t powerful enough to meet the energy demands of the future.
The International Energy Agency estimates that global battery capacity needs to increase sixfold by 2030.
Today’s batteries, whether they’re the disposable alkaline ones in our household gadgets or the rechargeable lithium-ion batteries in electric vehicles, use solid materials like metal oxides or graphite for their electrodes—the components through which ions move during charging and discharging.
But here’s the problem: every time a battery is charged or discharged, these solid materials expand and contract, sometimes by as much as 300%.
This constant expansion and contraction cause damage over time, which is why even rechargeable batteries eventually lose their capacity and die.
Detsi and his team are exploring new materials for batteries that can store large amounts of lithium, sodium, and magnesium, which are essential for high-performance batteries.
However, the more of these elements a battery material can hold, the more it expands and shrinks during use, leading to significant wear and tear.
Some researchers, including the late Nobel laureate John Goodenough, have started to develop batteries with liquid electrodes, which don’t crack under pressure.
But liquid electrodes come with their own set of challenges, like how to safely manufacture and use batteries that behave like water balloons.
This means that simply making bigger or liquid-based batteries won’t solve the problem. To create the batteries of the future, entirely new materials are needed.
Another issue is that the elements typically used in rechargeable batteries, like lithium and cobalt, are becoming more expensive and are often linked to human rights abuses. For example, cobalt, which is crucial for battery production, is largely sourced from the Democratic Republic of the Congo, where labor conditions are dire.
Detsi’s research focuses on creating batteries using more abundant and less ethically problematic materials like sodium and magnesium. These elements are plentiful, particularly in the U.S., where a large portion of the world’s sodium carbonate (used to make sodium) and sodium chloride (salt) reserves are found.
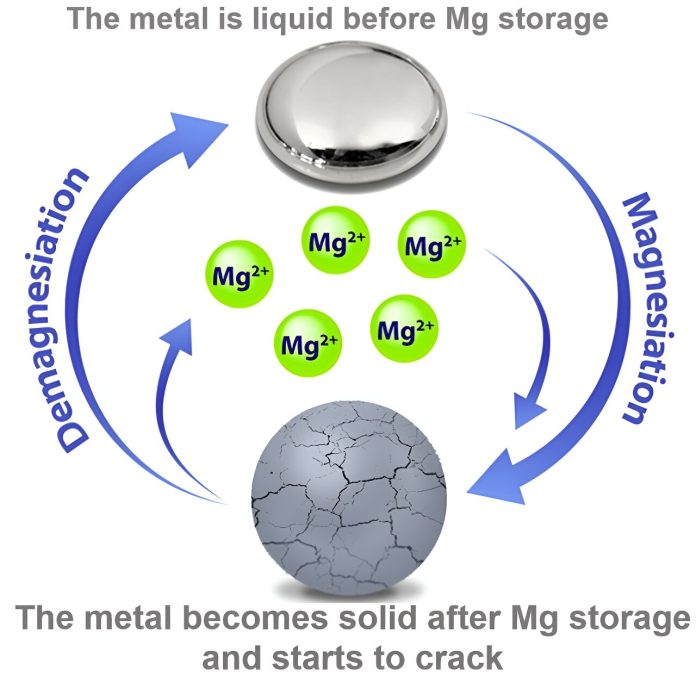
To solve the problem of battery degradation, Detsi’s group is developing electrodes that can shift between solid and liquid states. This allows the material to “heal” itself, avoiding damage during charge cycles while remaining easy to produce.
For instance, Detsi’s team demonstrated a self-healing battery using an anode made of a mixture of magnesium and gallium, which has a low melting point. This alloy can shift from solid to liquid, repairing itself during the charging process. In 2019, his lab showed that these self-healing anodes could last for over 1,000 charge cycles, five times longer than traditional magnesium-ion batteries.
Earlier this year, they went even further by using a gallium-indium anode that melts at room temperature, making it more suitable for commercial use.
This experimental anode survived 2,000 charging cycles while retaining 91% of its battery capacity, a significant improvement over current technology. For comparison, an iPhone 15 can handle 1,000 charging cycles while maintaining 80% of its capacity.
To better understand how these materials work, Detsi’s team used advanced imaging techniques like X-ray diffraction and cryogenic scanning electron microscopy, which allows them to study the self-healing process in detail.
Nearly a decade ago, when Detsi first proposed the idea of self-healing sodium- and magnesium-ion batteries, the concept wasn’t taken seriously. But today, with the rise of startups focused on sodium-ion batteries, his ideas are gaining traction.
This innovative research could pave the way for longer-lasting, more sustainable batteries that are crucial for our energy future.



