Lithium iron phosphate is a key material used in batteries for electric cars, energy storage systems, and tools.
Known for its long life, affordability, and safety, it’s a popular choice in battery technology.
However, these batteries often fall short of their theoretical energy storage capacity by up to 25%, leaving experts puzzled about the lost potential.
To unlock this hidden capacity, scientists need to understand where and how lithium ions are stored and released within the battery during charging and discharging.
Researchers at Graz University of Technology (TU Graz) have made significant progress in this area.
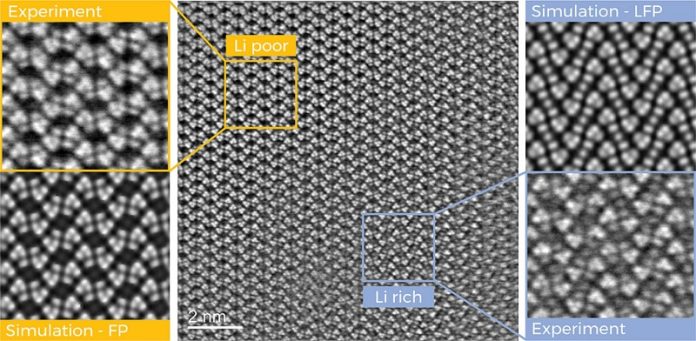
By using advanced transmission electron microscopes, they tracked the movement of lithium ions within the battery material and mapped their arrangement in the crystal structure of the battery’s iron phosphate cathode with unprecedented detail.
Their findings were published in the journal Advanced Energy Materials.
The research revealed that even when a battery is fully charged, some lithium ions remain trapped in the cathode instead of moving to the anode. These immobile ions reduce the battery’s overall capacity.
The researchers also discovered that these trapped ions are not evenly distributed within the cathode. They were able to pinpoint the exact locations of lithium enrichment and even separate these areas down to a few nanometers.
Interestingly, the study found that distortions and deformations occur in the crystal lattice of the cathode in these transition areas. These structural changes could be contributing to the loss of battery efficiency.
“This information is crucial for understanding the physical effects that limit battery performance,” said Ilie Hanzu, a researcher from TU Graz’s Institute of Chemistry and Technology of Materials. By taking these effects into account, scientists can work on improving the materials used in batteries.
The methods used in this study are not just limited to lithium iron phosphate batteries. The researchers believe that with minor adjustments, their techniques can be applied to other types of battery materials as well. This could help in developing better and more efficient batteries in the future.
Nikola Šimić, the study’s lead author, explained, “By combining different examination methods, we were able to determine where the lithium is positioned in the crystal channels and how it gets there.”
The knowledge gained from this study could lead to significant advancements in battery technology, making batteries more efficient and capable of storing more energy.
This discovery is an important step toward increasing the capacity of lithium-ion batteries, helping to meet the growing demand for more powerful and sustainable energy storage solutions.
Source: KSR.



