Lithium-ion batteries are widely used because they charge quickly, have high energy density, and can be recharged many times.
However, they have a major drawback: they are prone to short-circuiting.
This happens when a connection forms between the two electrodes inside the battery, leading to a sudden loss of voltage or a rapid discharge of high current.
In severe cases, a short circuit can cause the battery to overheat, catch fire, or even explode.
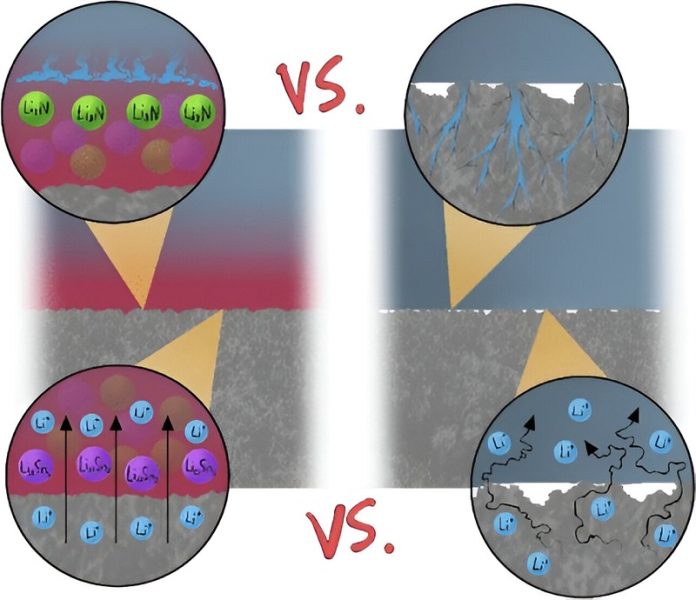
One of the main causes of short circuits is the growth of rough, tree-like structures called dendrites on the surface of one electrode.
These dendrites can grow across the cell and make contact with the other electrode, causing a short circuit.
Researchers from the University of Alberta (UAlberta) have found a promising way to prevent dendrites from forming in solid-state lithium-ion batteries.
Using the Canadian Light Source (CLS) at the University of Saskatchewan (USask), they discovered that adding a thin layer of tin between the electrode and the electrolyte helps distribute the lithium evenly when it is being deposited on the battery.
This creates a smooth surface that prevents the formation of dendrites.
Their findings were published in the journal ACS Applied Materials and Interfaces.
The researchers also found that the battery with the tin layer could operate at a higher current and endure more charging and discharging cycles than a regular battery.
Lingzi Sang, an assistant professor in UAlberta’s Faculty of Science (Chemistry), emphasized the importance of the CLS in their research. “The HXMA beamline allowed us to see at a structural level what was happening on the surface of the lithium in an operating battery,” says Sang.
“As a chemist, it was fascinating to observe the exact tin structure we introduced to the interface, which can suppress dendrites and solve the short-circuiting problem.”
In another study published earlier this year, the team showed that adding a protective layer of tin also prevented dendrites in liquid-electrolyte-based lithium-ion batteries.
This new method holds significant potential for industrial applications. “Our next step is to find a sustainable, cost-effective way to apply the protective layer during battery production,” adds Sang.
This breakthrough could lead to safer and more reliable lithium-ion batteries, benefiting various industries and everyday users alike. By preventing short circuits, these batteries could last longer and perform better, making them even more valuable in our daily lives.



