Seawater electrolysis, a process that can produce hydrogen from seawater, is a promising way to help the world reduce its reliance on fossil fuels.
Hydrogen is a clean energy source, but producing it sustainably has been challenging.
One of the main obstacles has been the difficulty in finding materials that can efficiently and affordably generate hydrogen from seawater without corroding or breaking down.
A new approach, developed by a team of researchers led by Professors Hong Chen, Bing-Jie Ni, and Zongping Shao, may have found a solution.
The team has created a new type of electrode that is both efficient and stable in seawater conditions, offering a significant advancement in the production of green hydrogen.
The research, published in Science Bulletin, focuses on using self-supported nickel-iron (NiFe) materials as catalysts.
These materials are known for their effectiveness and affordability in driving the chemical reactions needed to split water into hydrogen and oxygen.
However, previous attempts to use NiFe in seawater electrolysis have faced problems with corrosion and inefficiency.
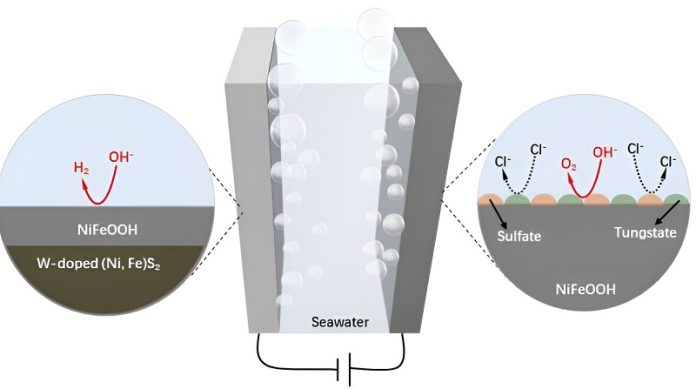
To overcome these issues, the researchers added tungsten to the NiFe-based catalysts. Tungsten helps protect the electrode from corrosion, making it more stable and durable.
They also used wood-based carbon (WC) structures as the foundation for these catalysts.
The WC structures have a porous design that improves the flow of electricity and enhances the overall efficiency of the electrode.
The result is a new W-NiFeS/WC electrode, which has a three-dimensional porous structure filled with tiny W-NiFeS particles.
This design increases the electrode’s surface area and boosts its ability to conduct electricity, leading to better performance in the electrolysis process.
The electrode also has rich redox-active centers, which are key to driving the reactions needed to produce hydrogen.
When tested in alkaline seawater, the new electrode outperformed traditional catalysts, showing superior activity and stability. It was particularly effective in both the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER), the two key processes in water splitting.
The innovative structure of the W-NiFeS/WC electrode allows it to resist corrosion, thanks to the formation of protective tungstate and sulfate layers during the reaction. Additionally, the electrode can efficiently catalyze the HER, making it a highly effective tool for producing hydrogen from seawater.
This breakthrough not only improves the efficiency of seawater electrolysis but also supports a circular economy approach. By using wood waste-derived carbon structures in the design, the research highlights a way to repurpose abundant waste materials, reducing environmental impact while advancing sustainable hydrogen production.
In summary, this new technology could play a crucial role in the future of green energy, offering a more sustainable and cost-effective method to produce hydrogen from seawater.



