Artificial intelligence (AI) is a powerful tool in research, but it often comes with a big challenge—explaining how it makes decisions.
This challenge is known as the “AI black box,” where the AI gives results without revealing how it got there.
For scientists, this lack of transparency can be frustrating, especially when trying to understand why certain materials work better than others.
Recently, a team of researchers from the University of Illinois Urbana-Champaign, along with a collaborator from the University of Toronto, found a way to peek inside this black box.
They combined AI with automated chemical experiments to discover new principles that make molecules better at harvesting solar energy.
Their breakthrough led to the creation of light-harvesting molecules that are four times more stable than the original ones.
This stability is crucial for making solar cells that last longer and perform better. The team also gained new insights into what makes these molecules stable—a question that has puzzled scientists for years.
The research team was made up of experts from various fields, including chemistry, chemical engineering, and materials science. They published their findings in the prestigious journal Nature.
One of the lead researchers, chemistry professor Nicholas Jackson, explained the problem with AI: “New AI tools are incredibly powerful. But when you try to understand what they’re doing, it’s usually hard to find anything useful.
AI can help us improve a molecule, but it can’t tell us why it’s better. Our process helped us figure out what makes these molecules more stable in light, turning the AI black box into a transparent glass globe.”
The researchers focused on improving organic solar cells, which are made from thin, flexible materials. These materials are different from the heavy, rigid silicon panels commonly used in solar power today. Organic solar cells have the potential to be cheaper and more versatile, but their stability has been a major issue since the 1980s. The materials tend to break down when exposed to light, which is a big problem for something meant to capture sunlight.
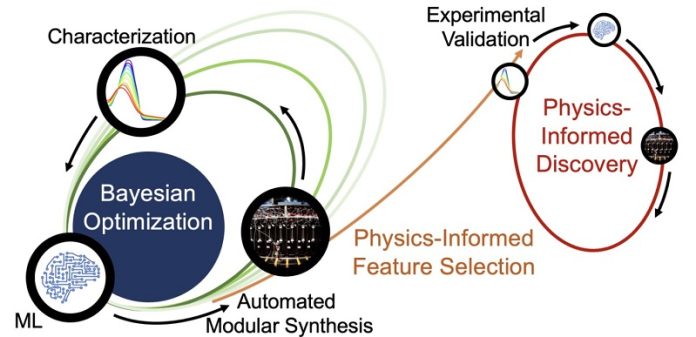
The team used a method called “closed-loop transfer,” which starts with an AI-guided process known as closed-loop experimentation. They asked the AI to find ways to make light-harvesting molecules more stable in light. The AI suggested different chemicals to try, and the researchers synthesized and tested these chemicals in several rounds. Each round produced better and more stable molecules.
Over five rounds of this process, the team created 30 new chemical candidates. They did this using a modular chemistry approach developed by chemistry professor Martin Burke’s group. This approach allows for quick and efficient synthesis of new molecules, which are then tested and fed back into the AI model. The AI gets smarter with each round, leading to even better results.
But the researchers didn’t stop there. They wanted to understand what made these new molecules so stable. While the experiments were running, another set of algorithms analyzed the molecules to find patterns and rules that predicted stability. These rules were then tested in the lab, confirming that the right chemical features could make molecules up to four times more stable in light.
This project is just the beginning. The researchers believe their method could be applied to other material systems, opening up new possibilities in science and technology. As professor Charles Schroeder put it, “The possibilities are only limited by our imagination.”
This work showcases the power of teamwork, as it brought together five different research groups to achieve something that no single team could have done alone. The collaboration between the universities and their resources made this groundbreaking discovery possible.



