Scientists from King’s College London and Imperial College London have made an exciting breakthrough in the fight against climate change.
They have successfully recreated the active site of an enzyme called Acetyl-CoA Synthase (ACS), which is involved in capturing carbon dioxide (CO2) from the atmosphere.
This discovery could lead to new ways to reduce CO2 levels and help combat global warming.
The research was led by Dr. Rebecca Musgrave from King’s College London’s Department of Chemistry and Dr. Daniel Wilson from University College London (UCL).
Their findings were published in the Journal of the American Chemical Society.
Acetyl-CoA Synthase is an enzyme that transforms CO2 into acetyl coenzyme-A, a molecule essential for life. ACS plays a key role in the Krebs Cycle, a series of chemical reactions in living organisms that produce energy.
This enzyme is crucial for both storing and releasing energy and capturing CO2 from the atmosphere.
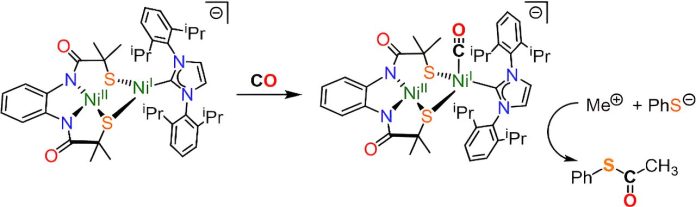
In their study, the team created a model of the ACS enzyme’s active site—the part of the enzyme where chemical reactions take place. This model was able to replicate the enzyme’s natural function in the lab, capturing CO2 from the air and converting it into acetyl coenzyme-A.
Enzymes are proteins that act as biological catalysts, speeding up chemical reactions. They perform many vital functions in nature, including human biology.
The chemical pathways created by enzymes have evolved over billions of years and are complex, making them challenging to study and replicate in the lab. To overcome this, scientists often create smaller models of enzyme active sites to study their functions.
ACS is found in bacteria and some single-celled organisms. It works without oxygen to build complex organic molecules from CO2 and hydrogen. Previous attempts to model the ACS enzyme’s active site in the lab failed to accurately replicate its shape and electronic environment needed for carbon capture.
Dr. Wilson explained that scientists have been studying the ACS enzyme for decades but struggled to understand how it produces acetyl coenzyme-A. In this study, the team developed a molecular model featuring two nickel atoms that mimics the ACS enzyme’s active site with remarkable accuracy.
When the team exposed their model to carbon monoxide, it successfully synthesized acetyl coenzyme-A, imitating the natural enzyme’s function. Working with Dr. Maxie Roessler at Imperial College, the researchers used a technique called Electron Paramagnetic Spectroscopy to study the reaction steps. This provides valuable insights for scientists studying ACS and other enzymes related to carbon capture.
Dr. Musgrave highlighted that their model opens new ways to understand how the ACS reaction works. By studying the reaction steps with advanced techniques, scientists can use this knowledge to design man-made catalysts for industrial use.
These catalysts could be used to capture CO2 from the atmosphere and convert it into useful carbon-based chemicals, such as biofuels or pharmaceuticals.
The researchers hope that their model will help other scientists studying enzyme spectroscopy. Dr. Musgrave emphasized that understanding how enzymes work so efficiently in nature could help design industrial-scale catalysts to tackle climate change and other societal issues.



