In an exciting development, researchers at Northwestern University have discovered that “dancing molecules” can regenerate damaged cartilage in just three days.
This groundbreaking therapy, which was initially designed to repair spinal cord injuries, has now been successfully applied to human cartilage cells.
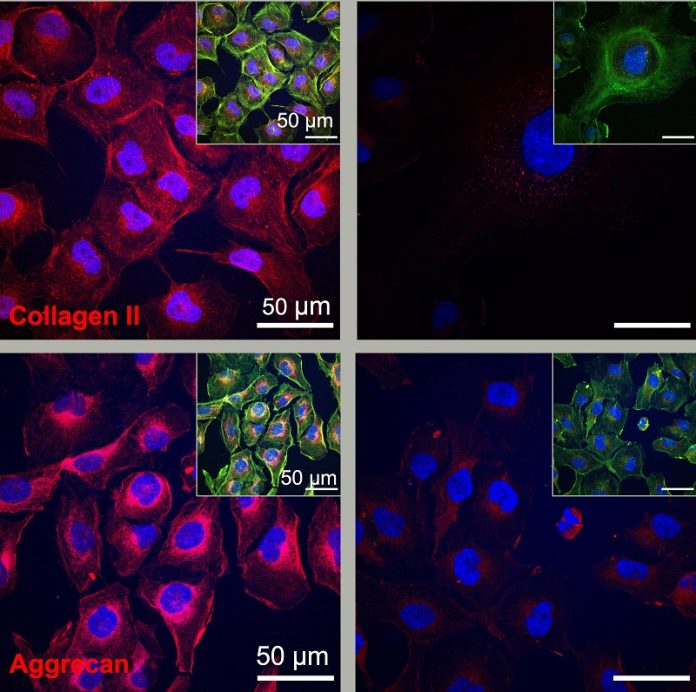
The study, published in the Journal of the American Chemical Society, shows that these fast-moving molecules can activate genes necessary for cartilage repair within hours and produce key proteins for regeneration within days.
The concept of “dancing molecules” was first introduced by the research group led by Samuel I. Stupp in November 2021.
These molecules move rapidly, enabling them to engage effectively with cell receptors.
By increasing their motion, the effectiveness of the treatment also increases, making the molecules’ movement a crucial factor in the cartilage growth process.
“When we saw the therapeutic effects of dancing molecules on spinal cord cells, we thought it could work for other types of cells as well,” said Stupp, who is a professor of materials science, engineering, chemistry, and medicine at Northwestern.
“Now we see similar effects on cartilage cells, suggesting we might have found a universal method for tissue regeneration.”
Stupp, an expert in regenerative nanomedicine, leads the Simpson Querrey Institute for BioNanotechnology at Northwestern. His team includes Shelby Yuan, a graduate student and the primary author of the study.
Osteoarthritis is a common and debilitating condition affecting nearly 530 million people worldwide. This degenerative disease causes the breakdown of joint tissues over time, leading to severe pain and impaired joint function. In advanced stages, the cartilage can wear away completely, causing bone-on-bone contact, which is extremely painful and often requires joint replacement surgery.
“Current treatments aim to slow the progression of the disease or delay the need for surgery, but they don’t regenerate cartilage,” Stupp said. “Humans lack the natural ability to regenerate cartilage in adulthood.”
The Science Behind Dancing Molecules
The idea behind “dancing molecules” is to encourage stubborn tissues to regenerate. These molecules form synthetic nanofibers that send potent signals to cells. By tuning their movements, the molecules can effectively engage with cell receptors, which are also in constant motion.
In this study, the researchers targeted a specific protein critical for cartilage formation called transforming growth factor beta-1 (TGFb-1). They developed a new circular peptide that mimics the bioactive signal of TGFb-1 and incorporated it into two different molecules forming supramolecular polymers. One polymer allowed the molecules to move freely, while the other restricted their movement.
“We modified the structure to compare the effects of different levels of molecular motion,” Stupp explained. “The polymer with more mobile molecules was much more effective.”
After three days, human cells exposed to the mobile molecules produced more proteins necessary for cartilage regeneration than those exposed to less mobile molecules. Remarkably, the dancing molecules were even more effective than the natural TGFb-1 protein in promoting cartilage growth.
Stupp’s team is now testing these systems in animal models and adding additional signals to enhance the therapy. “With these promising results, we believe that this therapy will greatly enhance cartilage regeneration in pre-clinical models,” Stupp said. “We aim to develop a novel bioactive material for joint cartilage repair.”
Additionally, the team is exploring the use of dancing molecules to regenerate bone and has already seen promising early results. They are also testing the molecules in human organoids to speed up the discovery of new therapeutic materials.
Stupp’s team is working towards gaining FDA approval for clinical trials to test the therapy for spinal cord repair. “We are starting to see the wide range of conditions that these dancing molecules could potentially treat,” Stupp said. “Controlling their motion through chemical design appears to be a powerful tool for a variety of regenerative therapies.”
This exciting research offers hope for new treatments for osteoarthritis and other conditions requiring tissue regeneration, potentially transforming the lives of millions of people.
If you care about wellness, please read studies about how ultra-processed foods and red meat influence your longevity, and why seafood may boost healthy aging.
For more health information, please see recent studies about the power of pickle juice ,and time-restricted eating: a simple way to fight aging and cancer.



