A recent study has made significant progress in understanding electron transfer (ET), a process where electrons move from one atom or molecule to another.
ET is essential in many fields, especially for creating advanced materials and improving electrochemical reactions.
However, the process of ET at the nanoscale (1–100 nanometers) in solids is not well understood.
Nanotubes, which are tiny cylindrical structures, have unique properties that make them ideal for studying nanoscale ET.
While carbon-based nanotubes are particularly interesting, they are difficult to control because their creation requires very high temperatures.
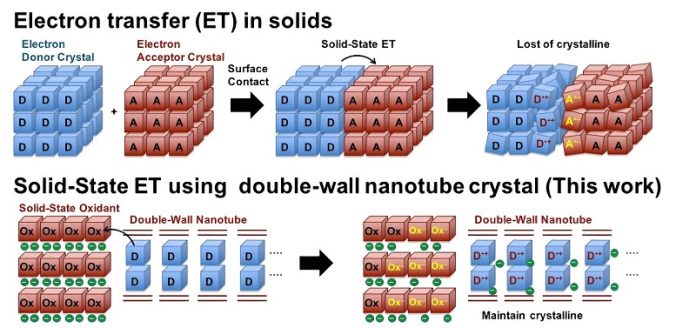
A new method involves making nanotubes from non-covalent interactions, where atoms are held together by weaker forces than in carbon nanotubes.
These nanotubes can be more precisely controlled but are usually not strong enough to handle the stress of electron transfers without breaking.
Researchers from Tokyo University of Science, led by Professor Junpei Yuasa, have developed a new type of nanotube that overcomes these challenges. They created crystalline nanotubes with a double-walled structure, making them strong and flexible enough to withstand ET processes. By adding electron donor molecules into these nanotubes, the team could observe the electron transfer directly.
The findings, published in Nature Communications on May 23, 2024, show that these double-walled nanotubes can maintain their structure during electron transfers. This was achieved using a technique called X-ray crystal structure analysis, which allowed the scientists to see the process in detail.
The researchers used a special method called supramolecular crystallization to create zinc-based double-walled nanotubes. These nanotubes have large openings in their walls, making them capable of absorbing electron donor molecules.
The team used ferrocene and tetrathiafulvalene as electron donors, which were absorbed into the nanotubes.
When electrons were removed from these donors through an oxidation reaction, the nanotubes remained intact, allowing for direct observation of the electron transfer.
This breakthrough is significant because understanding ET can lead to the development of better materials for electronic devices like semiconductors and transistors. It can also improve the performance of optoelectronic devices, such as solar cells, which rely heavily on efficient electron transfer.
Additionally, this research could advance energy storage technologies and other areas of nanotechnology and materials science.
In summary, this study provides a new way to observe and understand electron transfer in solid materials, potentially leading to many technological advancements.



