Researchers from Trinity College Dublin’s School of Natural Sciences have discovered a new way to form bastnäsite, a key mineral used to extract rare earth elements (REEs).
This breakthrough could lead to more efficient methods for obtaining these valuable elements, which are crucial for technologies like smartphones and renewable energy solutions.
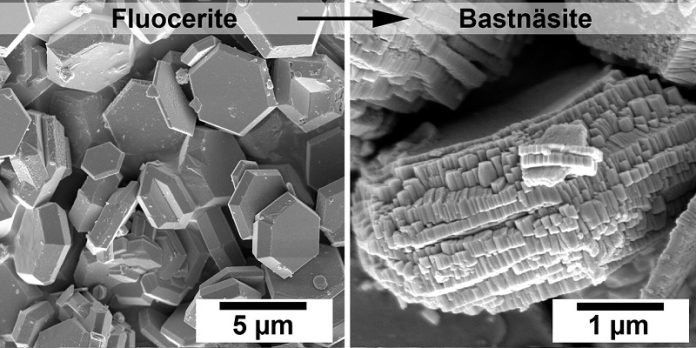
The study, published in the journal Nanoscale, reveals for the first time how the rare mineral fluocerite quickly forms and then transforms into bastnäsite.
Understanding fluocerite’s occurrence and origin has been a challenge for earth scientists because it’s hard to study in natural samples.
The Trinity team has found a new crystallization route that produces extremely tiny minerals, some just a few billionths of a meter in size.
These nanometric minerals are very difficult to observe in nature, but the researchers discovered that fluocerite can act as a “seed” to promote the rapid formation of bastnäsite.
This new knowledge not only advances scientific understanding but also has practical implications. It could lead to more efficient and cost-effective methods for extracting REEs, which are vital for a wide range of technologies.
Dr. Luca Terribili, the lead author of the study, explained that earth scientists have struggled to understand all the factors controlling the formation of bastnäsite. “We have shown for the first time that fluocerite can turn into bastnäsite.
This discovery was made by building synthetic bastnäsite rocks in the laboratory to mimic the natural processes and studying them with powerful spectroscopic and microscopic techniques,” said Dr. Terribili.
This innovative approach helped clarify the complex natural processes involved in the formation of these tiny minerals and paves the way for more efficient extraction of REEs.
Professor Juan Diego Rodriguez-Blanco, the principal investigator, added that the study highlights how these transformations can occur at relatively low temperatures and very quickly.
“These insights are crucial for developing better industrial methods for extracting rare earth elements. The reaction that turns fluocerite to bastnäsite may seem slow, taking between five hours and a month depending on temperature, but it is very rapid in geological timescales,” he said.
This research offers a promising future for making REE extraction more efficient, which is essential for various high-tech applications and green technologies. By better understanding the formation of bastnäsite, scientists can develop new techniques to improve the supply of these critical materials.
Overall, this study not only advances our scientific knowledge but also has significant practical implications, potentially transforming how we extract and utilize rare earth elements in the future.



