Scientists at EPFL have made a significant breakthrough in carbon capture technology by developing advanced atom-thin graphene membranes.
These new membranes, enhanced with pyridinic nitrogen at the pore edges, show exceptional performance in capturing carbon dioxide (CO2), marking a major step towards more efficient and cost-effective carbon capture solutions.
As the world faces the urgent challenge of climate change, reducing industrial carbon emissions has become a critical goal.
Carbon capture, utilization, and storage (CCUS) is a key technology aimed at capturing CO2 emissions from major industrial sources such as power plants, cement factories, steel mills, and waste incinerators.
However, current carbon capture methods are energy-intensive and expensive, limiting their widespread use.
To improve the efficiency and reduce the cost of carbon capture, scientists are developing membranes that can selectively capture CO2.
Existing membranes, like polymer thin films, struggle with limited CO2 permeance (how well CO2 passes through) and selectivity (how well the membrane separates CO2 from other gases), making them less effective for large-scale applications.
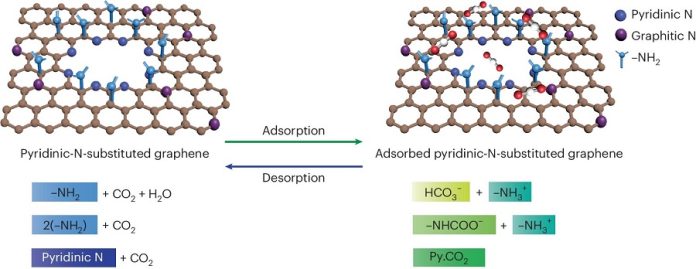
Led by Kumar Varoon Agrawal, the EPFL team has developed a new type of membrane using graphene, a material known for its strength and thinness.
They incorporated pyridinic nitrogen at the edges of the graphene pores, significantly enhancing the membrane’s ability to capture CO2.
The researchers started by creating single-layer graphene films using chemical vapor deposition on copper foil. They introduced pores into the graphene through controlled oxidation with ozone, forming oxygen-atom functionalized pores.
Then, they reacted the oxidized graphene with ammonia at room temperature to add nitrogen atoms at the pore edges in the form of pyridinic nitrogen.
Using techniques like X-ray photoelectron spectroscopy and scanning tunneling microscopy, the team confirmed the successful addition of pyridinic nitrogen and the formation of CO2-binding complexes at the pore edges.
This addition greatly improved the binding of CO2 to the graphene pores.
The new graphene membranes demonstrated a high CO2/N2 separation factor, averaging 53 for gas streams containing 20% CO2. For gas streams with about 1% CO2, the membranes achieved separation factors above 1,000 due to the effective and reversible binding of CO2 at the pore edges, facilitated by the pyridinic nitrogen.
This breakthrough in membrane technology could lead to more efficient and cost-effective carbon capture methods, which are essential for reducing industrial CO2 emissions and combating climate change.
The high performance of these graphene membranes makes them highly promising for various industrial applications, potentially revolutionizing the field of carbon capture and storage.
The research, published in Nature Energy, highlights the potential of advanced materials like graphene in creating sustainable and scalable solutions for global environmental challenges.



