Hydrogen (H2) is a promising fuel that can help reduce greenhouse gases, especially if it’s produced by splitting water (H2O) using renewable energy.
While this may sound simple, the chemistry involved is quite complex.
Breaking water into hydrogen and oxygen requires two separate electrochemical reactions, each needing a catalyst to help break and reform chemical bonds.
Now, scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Columbia University have developed a new, efficient catalyst for the harder part: the oxygen evolution reaction.
Published in the Journal of the American Chemical Society, the new catalyst was designed based on theoretical calculations.
The goal was to minimize the amount of iridium, an expensive metal used in catalysts, and to maximize the catalyst’s stability in acidic conditions.
The team created models of the catalyst and tested them in the lab, confirming their predictions.
They then made a powder form of the catalyst, similar to those used in industrial applications, and showed it can efficiently produce hydrogen in a water-splitting device.
“In real-world tests, our catalyst is about four times better than the state-of-the-art commercially available iridium catalyst,” said Jingguang Chen, a chemical engineer at Columbia University who led the research.
This means the new catalyst requires four times less iridium to produce hydrogen at the same rate as the commercial version or produces hydrogen four times faster for the same amount of iridium.
Ping Liu, a theoretical chemist at Brookhaven Lab who led the calculations for the catalyst’s design, said, “This study shows how you can go from understanding what’s happening at the atomic level to designing a practical catalyst. Our work gives us a better understanding of how this catalyst works and gets us closer to real-world application.”
One remaining challenge is to scale up production. “We are only making milligrams of catalyst per batch,” Chen said. “To produce megatons of green hydrogen, we’d need kilograms or tons of catalyst. We can’t make it at that large scale yet.”
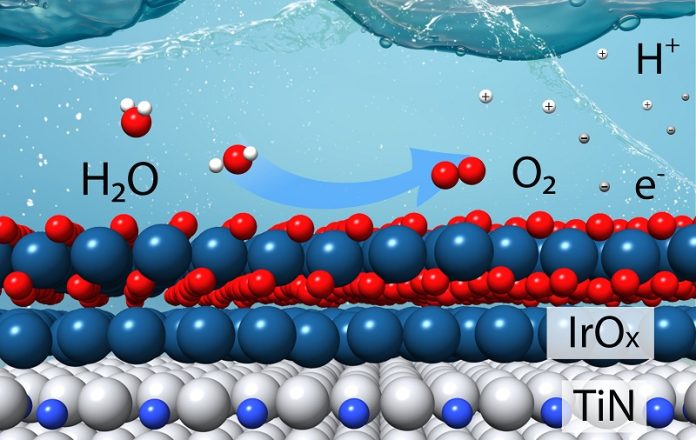
Iridium is currently the catalyst of choice for the oxygen evolution reaction, which happens at the anode of an electrolyzer. It provides the electrically charged active sites that separate tightly bound hydrogen ions (H+) from oxygen (O).
This reaction also produces oxygen gas (O2) and electrons, which are needed for the second, less challenging “hydrogen evolution” reaction.
“Iridium is one of the only stable elements for the oxygen evolution reaction in acid,” Chen said. However, iridium is rare and more expensive than platinum, making it crucial to reduce the amount used.
The team explored using earth-abundant elements like titanium combined with nitrogen, which proved stable in acidic conditions. They reasoned that a catalyst could be made of a less-expensive material with iridium only on the surface.
Using density functional theory calculations, they modeled how different layers of iridium on titanium nitride would affect the catalyst’s stability and activity under acidic conditions. The calculations showed that two or three layers of iridium would work best.
The team then created thin films and powdered samples of the catalyst and studied them using various techniques, including electron microscopy and X-ray spectroscopy. The studies confirmed that iridium and titanium interact strongly, stabilizing the iridium and improving the reaction.
“Our findings show that the interaction between iridium and titanium is crucial for both stability and activity,” Liu said. “This study provides guidelines industrial chemists could use to make true core-shell structures with a uniform thin layer of iridium.”
Such catalysts could help lower the cost of water splitting and bring us closer to producing large quantities of green hydrogen, potentially transforming the energy landscape.



