Hydrogen is a clean fuel that can help reduce our reliance on fossil fuels and decrease carbon dioxide emissions.
However, most of the hydrogen we use today is made from methane, a fossil fuel, through a process called methane reforming.
This process produces a lot of carbon dioxide, which contributes to climate change.
To make hydrogen production greener, we need better, scalable alternatives.
One promising method for producing green hydrogen is water electrolysis.
This process uses electricity to split water into hydrogen and oxygen. If the electricity comes from renewable sources, the hydrogen produced is truly green.
Water electrolysis requires catalysts at both the cathode and anode to speed up the otherwise slow reactions.
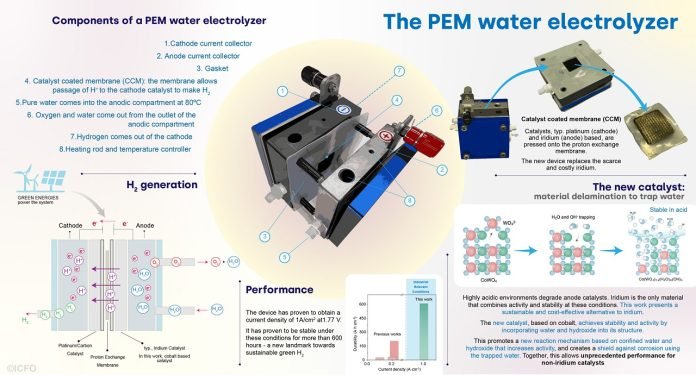
One of the best types of water electrolysis is called proton-exchange-membrane (PEM), which combines high rates of hydrogen production with high energy efficiency.
Until now, PEM water electrolysis has relied on catalysts made from rare and expensive elements like platinum and iridium. These elements are used because they can withstand the harsh, acidic conditions of the reaction.
This is particularly true for anode catalysts, where iridium oxides are commonly used due to their stability.
However, iridium is one of the rarest elements on Earth, making it an unsustainable option for large-scale hydrogen production.
A team of scientists has made a significant breakthrough in finding alternatives to iridium catalysts. This team, led by Professor F. Pelayo García de Arquer at ICFO, developed a new way to make an iridium-free catalyst stable under industrial conditions. Their discovery, published in the journal Science, marks the first time a catalyst has been shown to work in PEM water electrolysis without using iridium.
The key challenge was to create a catalyst that combines both activity and stability in a highly acidic environment. Most materials tend to dissolve in such conditions, making it difficult to find a stable alternative to iridium.
Previous research had explored using materials like manganese and cobalt oxides, but these studies were often conducted under less demanding conditions, making them unsuitable for industrial applications.
The breakthrough came from thinking outside the box. Instead of just changing the composition or structure of the catalyst materials, the researchers incorporated water and its fragments directly into the catalyst’s structure.
This innovative approach, using abundant and inexpensive cobalt, allowed the catalyst to operate stably in acidic conditions at the high current densities required for industrial use.
Professor García de Arquer explained, “Conventional catalyst design focuses on altering the materials used. We took a different approach by designing a new material that involves water and its fragments in its structure. This shields the catalyst under challenging conditions, enabling stable operation at high current densities.”
Their technique involved a delamination process, which exchanged part of the material for water, creating a stable, efficient, and iridium-free catalyst. This discovery opens the door to more sustainable and scalable green hydrogen production, reducing our reliance on rare and expensive elements.
In summary, this new catalyst represents a major step forward in the quest for green hydrogen. By harnessing the hidden power of water, scientists have developed a stable and efficient alternative to iridium-based catalysts, paving the way for a cleaner and more sustainable future.
Source: KSR.



