Researchers have developed an exciting new way to produce electricity using the natural mixing of river water and sea water, a method known as “blue” osmotic energy.
This discovery was reported in the scientific journal ACS Energy Letters.
Estuaries, where rivers flow into the sea, are not only beautiful places for birdwatching and kayaking but also natural spots where waters of different salt levels blend.
This difference in salt content can be used to generate sustainable energy.
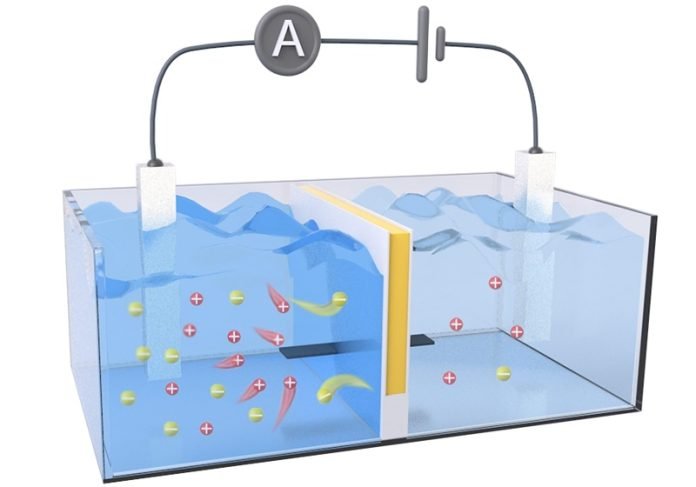
The key to this process is a special kind of battery made from layers of a material called a membrane.
These membranes act like filters that allow certain particles, like salt ions, to pass through while blocking others.
When freshwater from a river meets salty sea water, it creates a natural pressure as the salt ions try to spread out evenly. This pressure can be converted into electricity.
A team led by researchers Dongdong Ye and Xingzhen Qin crafted a new type of membrane that works more efficiently than those currently available.
Their innovative design separates the paths for salt ions and electricity to travel through the membrane, which helps generate more power.
This separation is achieved by placing a gel material that attracts ions between two layers of a substance that conducts electricity.
In laboratory tests, this new membrane design generated more than twice the power of existing commercial membranes. To test their prototype in conditions that mimic nature, the researchers used a water tank designed like an estuary.
The results were promising: the new membrane worked continuously for over two weeks without losing effectiveness and managed to generate enough power to run a calculator, an LED light, and a stopwatch.
This breakthrough suggests that similar membranes could be developed using environmentally friendly materials, improving the ability to harvest osmotic energy.
This type of energy generation is particularly appealing because it uses the natural environment and does not rely on burning fossil fuels, making it a cleaner and more sustainable option.
Overall, this advancement could lead to more practical applications of osmotic energy, bringing us closer to harnessing the vast power of our waterways.
The work of Ye, Qin, and their colleagues opens up new possibilities for using natural salt differences in water to power our everyday devices.



