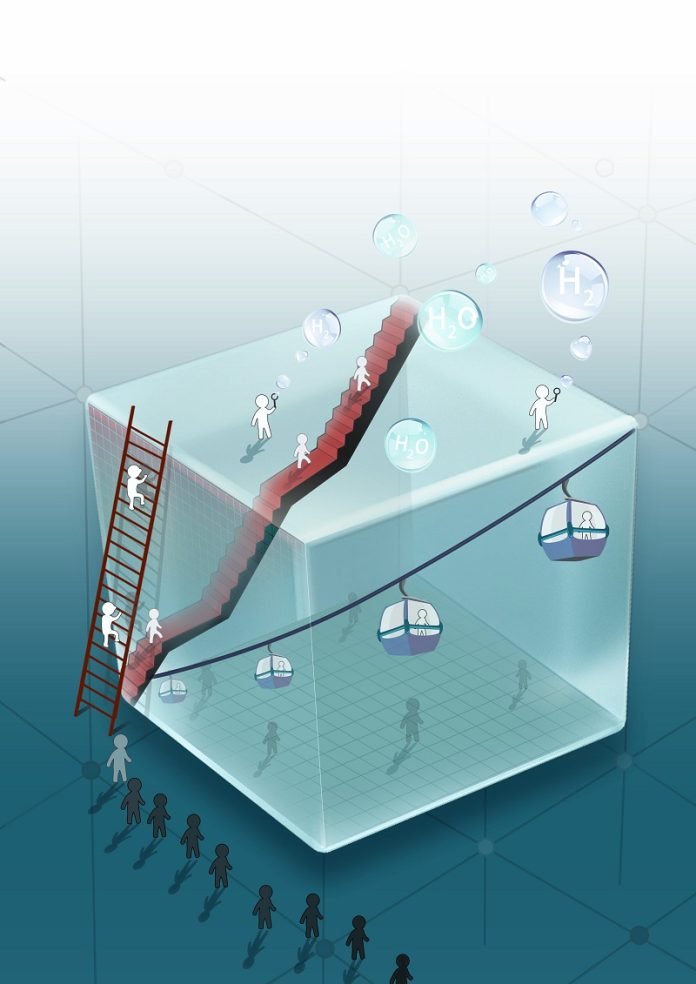
Researchers from the University of Cambridge have made a significant breakthrough in generating clean hydrogen fuel from water using sunlight.
Their innovative approach involves a simple yet effective twist in the design of copper oxide semiconductors, commonly used materials that are affordable, abundant, and non-toxic.
Typically, copper oxides don’t perform as well as silicon, which is widely used in solar technologies.
However, the Cambridge team discovered that by altering the way copper oxide crystals grow—orienting them so that electric charges travel diagonally—they could substantially speed up and extend the movement of these charges, leading to a 70% increase in efficiency compared to the best existing copper oxide devices.
This new method not only enhances the performance of copper oxide light harvesters, known as photocathodes, but also significantly improves their stability.
This advancement was detailed in the prestigious journal Nature, where the team highlighted the potential of low-cost materials in moving away from fossil fuels towards sustainable energy solutions.
Copper (I) oxide, or cuprous oxide, has been considered a promising alternative to silicon for solar energy applications due to its effectiveness in capturing sunlight and converting it into electrical charge.
However, it has historically faced challenges, primarily because much of the electric charge generated tends to get lost within the material, limiting its overall efficiency.
Dr. Linfeng Pan, co-first author of the study, explained that the new method focuses on how deep light is absorbed and how far charges can travel within the material, aiming to utilize more than just the surface layer.
Professor Sam Stranks, who led the research, noted that while most solar materials suffer from surface defects, with oxide materials, the challenges lie deeper within the bulk of the material, making the growth process crucial to their effectiveness.
The team’s approach involves growing cuprous oxide films using thin film deposition techniques, which can be performed at ambient pressure and room temperature.
By precisely controlling the growth conditions, they were able to align the crystals in a specific orientation that allowed for better movement of electric charges.
These insights were verified through high temporal resolution spectroscopic techniques, which showed that when electrons move diagonally through the crystal structure, they travel much farther, significantly boosting the material’s performance.
The development of this technology marks a substantial improvement in the potential applications of copper oxides for solar energy conversion.
The researchers are optimistic about further enhancing these materials and believe that their findings could play a crucial role in the transition to renewable energy sources.
While more research is needed to fully understand and optimize these materials, the team is excited about the future possibilities.
“There’s still a long way to go, but we’re on an exciting trajectory,” said Professor Stranks, emphasizing the promising future of this technology in clean energy generation.