Researchers at the University of Virginia have made a groundbreaking discovery in clean energy technology.
In their quest to find more sustainable and affordable alternatives to costly metal catalysts used in fuel cells, they have identified an organic molecule that could do the job just as effectively but at a lower cost.
Fuel cells are crucial in powering electric vehicles and supporting the operation of industrial and residential generators.
These cells are also integral in storing energy from renewable sources like wind and solar. Traditionally, metals such as platinum are used to catalyze the chemical reactions in fuel cells that convert hydrogen gas into electricity.
However, these metals are not only rare but also expensive.
The discovery, published in the Journal of the American Chemical Society, comes from the combined efforts of associate professors of chemistry Charles Machan, Michael Hilinski, and their Ph.D. students, Emma Cook and Anna Davis.
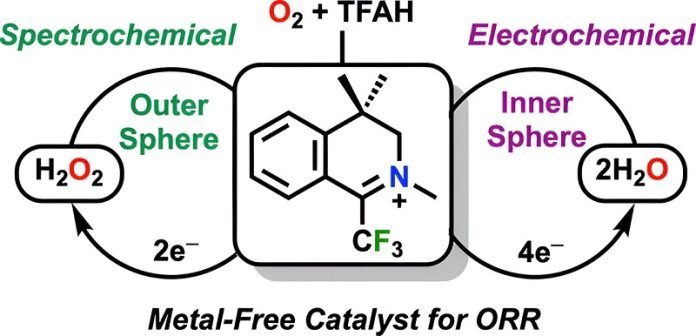
They found that a newly identified organic molecule made of carbon, hydrogen, nitrogen, and fluorine is capable of catalyzing the oxygen reduction reaction in fuel cells—an essential process currently facilitated by platinum.
What makes this molecule particularly noteworthy is its ability to initiate and sustain the reaction without breaking down, a common issue with other organic catalysts.
According to Professor Machan, this molecule demonstrates stability and activity that rivals traditional metal catalysts and can repeatedly return to its original state after reacting, allowing for ongoing reaction cycles without degradation.
While this specific molecule might not end up in commercial fuel cells, its discovery opens the door to the development of carbon-based catalytic materials that could potentially replace expensive metals.
The team’s long-term goal is to integrate the properties of this stable molecule into a bulk material that could one day be used widely in fuel cells.
The interdisciplinary nature of the research team, combining expertise in organic chemistry and molecular electrochemistry, was crucial to this discovery.
Hilinski’s background in studying the molecule in other chemical reactions laid the groundwork, while Machan’s focus on its application in fuel cell chemistry propelled their research into new territory.
Besides the potential to revolutionize fuel cell technology, this discovery could also impact the production of hydrogen peroxide, a common household and industrial chemical.
The current production process of hydrogen peroxide is energy-intensive and environmentally unfriendly. The organic molecule identified by the University of Virginia team could improve the efficiency and environmental impact of this process, benefiting industries like paper production and wastewater treatment.
Furthermore, the electrification of the catalyst has changed its reactive properties in unexpected ways, which the team believes could be beneficial for synthesizing pharmaceuticals.
This aspect is something Hilinski’s research group is particularly excited to explore further.
This breakthrough not only represents a significant advance in the search for more sustainable and cost-effective energy solutions but also illustrates the power of interdisciplinary collaboration in tackling complex scientific challenges.
Source: University of Virginia.